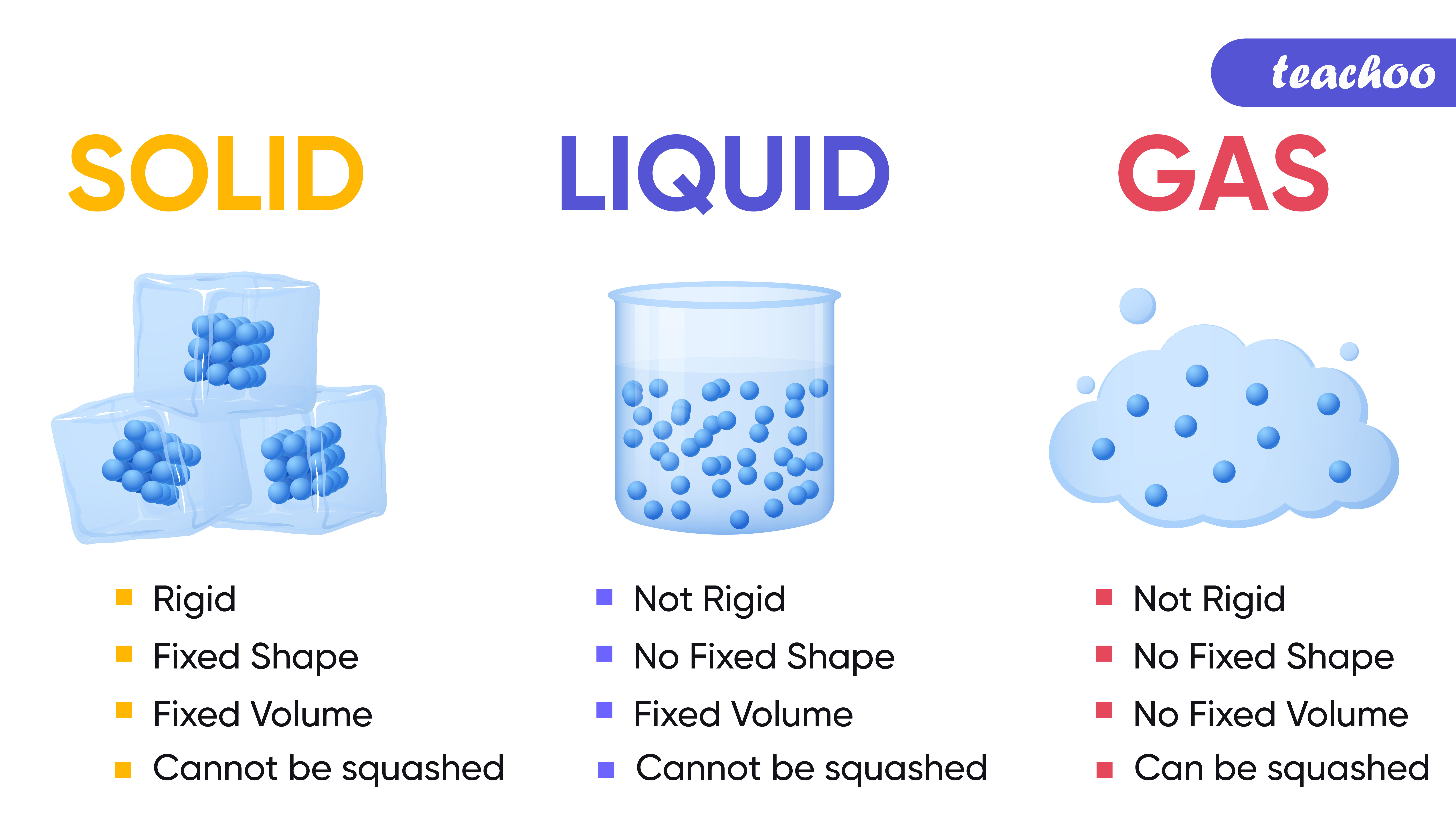
The Difference Between Solids/Liquids/Gases On A Molecular Level . There are three different states of matter: A solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. This model explains the higher density, greater order, and lower compressibility of liquids versus gases; Generally, the strength of the. What determines the phase of a substance? The solid, the liquid, and the gas phase. The thermal expansion of liquids; Gases, liquids and solids are all made up of atoms, molecules, and/or ions, but the behaviors of these particles differ in the three phases. Figure \(\pageindex{2}\) shows the differences among solids, liquids, and gases at the molecular level. In all these states molecules, atoms, or ions are in motion with a specific. Let us begin at the. This model explains the higher density, greater order, and lower compressibility of liquids versus gases;
from circuitengineverbis77.z13.web.core.windows.net
Generally, the strength of the. There are three different states of matter: What determines the phase of a substance? A solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. Figure \(\pageindex{2}\) shows the differences among solids, liquids, and gases at the molecular level. Gases, liquids and solids are all made up of atoms, molecules, and/or ions, but the behaviors of these particles differ in the three phases. Let us begin at the. This model explains the higher density, greater order, and lower compressibility of liquids versus gases; This model explains the higher density, greater order, and lower compressibility of liquids versus gases; The solid, the liquid, and the gas phase.
Solid Liquid And Gas Particle Diagram
The Difference Between Solids/Liquids/Gases On A Molecular Level There are three different states of matter: Gases, liquids and solids are all made up of atoms, molecules, and/or ions, but the behaviors of these particles differ in the three phases. Generally, the strength of the. There are three different states of matter: A solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. Figure \(\pageindex{2}\) shows the differences among solids, liquids, and gases at the molecular level. The thermal expansion of liquids; This model explains the higher density, greater order, and lower compressibility of liquids versus gases; In all these states molecules, atoms, or ions are in motion with a specific. The solid, the liquid, and the gas phase. Let us begin at the. What determines the phase of a substance? This model explains the higher density, greater order, and lower compressibility of liquids versus gases;
From lessonfullantje.z19.web.core.windows.net
Illustration Of Solid Liquid And Gas The Difference Between Solids/Liquids/Gases On A Molecular Level In all these states molecules, atoms, or ions are in motion with a specific. There are three different states of matter: Let us begin at the. The solid, the liquid, and the gas phase. What determines the phase of a substance? A solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a. The Difference Between Solids/Liquids/Gases On A Molecular Level.
From mungfali.com
Solids Liquids Gases Chart The Difference Between Solids/Liquids/Gases On A Molecular Level This model explains the higher density, greater order, and lower compressibility of liquids versus gases; A solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. What determines the phase of a substance? The thermal expansion of liquids; Generally, the strength of the. This. The Difference Between Solids/Liquids/Gases On A Molecular Level.
From www.visionlearning.com
Properties of Liquids Chemistry Visionlearning The Difference Between Solids/Liquids/Gases On A Molecular Level The solid, the liquid, and the gas phase. The thermal expansion of liquids; Gases, liquids and solids are all made up of atoms, molecules, and/or ions, but the behaviors of these particles differ in the three phases. This model explains the higher density, greater order, and lower compressibility of liquids versus gases; There are three different states of matter: Generally,. The Difference Between Solids/Liquids/Gases On A Molecular Level.
From www.tutorix.com
Give three characteristics of solid liquid and gas Tutorix The Difference Between Solids/Liquids/Gases On A Molecular Level There are three different states of matter: This model explains the higher density, greater order, and lower compressibility of liquids versus gases; A solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. In all these states molecules, atoms, or ions are in motion. The Difference Between Solids/Liquids/Gases On A Molecular Level.
From www.stuvia.com
The Three States of Matter Understanding the Properties and The Difference Between Solids/Liquids/Gases On A Molecular Level This model explains the higher density, greater order, and lower compressibility of liquids versus gases; Let us begin at the. Figure \(\pageindex{2}\) shows the differences among solids, liquids, and gases at the molecular level. The thermal expansion of liquids; A solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has. The Difference Between Solids/Liquids/Gases On A Molecular Level.
From www.pinterest.ph
Properties of Solids, Liquids, Gases Compared Teachoo Science The Difference Between Solids/Liquids/Gases On A Molecular Level A solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. The solid, the liquid, and the gas phase. Figure \(\pageindex{2}\) shows the differences among solids, liquids, and gases at the molecular level. What determines the phase of a substance? The thermal expansion of. The Difference Between Solids/Liquids/Gases On A Molecular Level.
From byjus.com
Draw a sketch to show the arrangement of particles in solid, liquid and The Difference Between Solids/Liquids/Gases On A Molecular Level The solid, the liquid, and the gas phase. Gases, liquids and solids are all made up of atoms, molecules, and/or ions, but the behaviors of these particles differ in the three phases. This model explains the higher density, greater order, and lower compressibility of liquids versus gases; Figure \(\pageindex{2}\) shows the differences among solids, liquids, and gases at the molecular. The Difference Between Solids/Liquids/Gases On A Molecular Level.
From www.pinterest.com
States of Matter solids, liquids and gases Chemistry for All The The Difference Between Solids/Liquids/Gases On A Molecular Level This model explains the higher density, greater order, and lower compressibility of liquids versus gases; A solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. There are three different states of matter: Let us begin at the. Generally, the strength of the. Figure. The Difference Between Solids/Liquids/Gases On A Molecular Level.
From www.slideserve.com
PPT SOLIDS LIQUIDS GASES PowerPoint Presentation, free download ID The Difference Between Solids/Liquids/Gases On A Molecular Level Gases, liquids and solids are all made up of atoms, molecules, and/or ions, but the behaviors of these particles differ in the three phases. Figure \(\pageindex{2}\) shows the differences among solids, liquids, and gases at the molecular level. There are three different states of matter: The thermal expansion of liquids; The solid, the liquid, and the gas phase. Let us. The Difference Between Solids/Liquids/Gases On A Molecular Level.
From www.youtube.com
Difference Between Solid Liquid And Gases State Of Matter The Difference Between Solids/Liquids/Gases On A Molecular Level Generally, the strength of the. This model explains the higher density, greater order, and lower compressibility of liquids versus gases; Figure \(\pageindex{2}\) shows the differences among solids, liquids, and gases at the molecular level. There are three different states of matter: A solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a. The Difference Between Solids/Liquids/Gases On A Molecular Level.
From www.numerade.com
SOLVED Draw molecularlevel views that show the differences among The Difference Between Solids/Liquids/Gases On A Molecular Level Figure \(\pageindex{2}\) shows the differences among solids, liquids, and gases at the molecular level. The solid, the liquid, and the gas phase. This model explains the higher density, greater order, and lower compressibility of liquids versus gases; This model explains the higher density, greater order, and lower compressibility of liquids versus gases; Gases, liquids and solids are all made up. The Difference Between Solids/Liquids/Gases On A Molecular Level.
From www.jove.com
11340.jpg The Difference Between Solids/Liquids/Gases On A Molecular Level Figure \(\pageindex{2}\) shows the differences among solids, liquids, and gases at the molecular level. Gases, liquids and solids are all made up of atoms, molecules, and/or ions, but the behaviors of these particles differ in the three phases. In all these states molecules, atoms, or ions are in motion with a specific. A solid has definite volume and shape, a. The Difference Between Solids/Liquids/Gases On A Molecular Level.
From vdocuments.mx
Aim What is the difference between solids, liquids, and gases? [PPTX The Difference Between Solids/Liquids/Gases On A Molecular Level Gases, liquids and solids are all made up of atoms, molecules, and/or ions, but the behaviors of these particles differ in the three phases. The solid, the liquid, and the gas phase. Figure \(\pageindex{2}\) shows the differences among solids, liquids, and gases at the molecular level. What determines the phase of a substance? A solid has definite volume and shape,. The Difference Between Solids/Liquids/Gases On A Molecular Level.
From sebschemistry.blogspot.com
IGCSE Edexcel Chemistry Help 1.1 understand the arrangement, movement The Difference Between Solids/Liquids/Gases On A Molecular Level Figure \(\pageindex{2}\) shows the differences among solids, liquids, and gases at the molecular level. This model explains the higher density, greater order, and lower compressibility of liquids versus gases; Gases, liquids and solids are all made up of atoms, molecules, and/or ions, but the behaviors of these particles differ in the three phases. The thermal expansion of liquids; Generally, the. The Difference Between Solids/Liquids/Gases On A Molecular Level.
From guidewiringdietician.z5.web.core.windows.net
3 States Of Matter Diagram The Difference Between Solids/Liquids/Gases On A Molecular Level Gases, liquids and solids are all made up of atoms, molecules, and/or ions, but the behaviors of these particles differ in the three phases. There are three different states of matter: This model explains the higher density, greater order, and lower compressibility of liquids versus gases; Let us begin at the. The thermal expansion of liquids; This model explains the. The Difference Between Solids/Liquids/Gases On A Molecular Level.
From circuitengineverbis77.z13.web.core.windows.net
Solid Liquid And Gas Particle Diagram The Difference Between Solids/Liquids/Gases On A Molecular Level Gases, liquids and solids are all made up of atoms, molecules, and/or ions, but the behaviors of these particles differ in the three phases. Figure \(\pageindex{2}\) shows the differences among solids, liquids, and gases at the molecular level. Let us begin at the. This model explains the higher density, greater order, and lower compressibility of liquids versus gases; What determines. The Difference Between Solids/Liquids/Gases On A Molecular Level.
From www.alamy.com
matter in different states for example water. solid, liquid, gas and The Difference Between Solids/Liquids/Gases On A Molecular Level This model explains the higher density, greater order, and lower compressibility of liquids versus gases; Generally, the strength of the. The solid, the liquid, and the gas phase. The thermal expansion of liquids; Let us begin at the. Gases, liquids and solids are all made up of atoms, molecules, and/or ions, but the behaviors of these particles differ in the. The Difference Between Solids/Liquids/Gases On A Molecular Level.
From www.animalia-life.club
Examples Of Solids Liquids And Gases The Difference Between Solids/Liquids/Gases On A Molecular Level Let us begin at the. There are three different states of matter: What determines the phase of a substance? The thermal expansion of liquids; The solid, the liquid, and the gas phase. This model explains the higher density, greater order, and lower compressibility of liquids versus gases; Generally, the strength of the. Gases, liquids and solids are all made up. The Difference Between Solids/Liquids/Gases On A Molecular Level.
From www.britannica.com
solid Definition & Facts Britannica The Difference Between Solids/Liquids/Gases On A Molecular Level What determines the phase of a substance? There are three different states of matter: In all these states molecules, atoms, or ions are in motion with a specific. A solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. Generally, the strength of the.. The Difference Between Solids/Liquids/Gases On A Molecular Level.
From saylordotorg.github.io
Solids, Liquids, and Gases The Difference Between Solids/Liquids/Gases On A Molecular Level Figure \(\pageindex{2}\) shows the differences among solids, liquids, and gases at the molecular level. Generally, the strength of the. The solid, the liquid, and the gas phase. There are three different states of matter: What determines the phase of a substance? This model explains the higher density, greater order, and lower compressibility of liquids versus gases; Let us begin at. The Difference Between Solids/Liquids/Gases On A Molecular Level.
From www.pinterest.com
difference between solid liquid and gas Google Search in 2021 The Difference Between Solids/Liquids/Gases On A Molecular Level This model explains the higher density, greater order, and lower compressibility of liquids versus gases; The thermal expansion of liquids; Figure \(\pageindex{2}\) shows the differences among solids, liquids, and gases at the molecular level. This model explains the higher density, greater order, and lower compressibility of liquids versus gases; In all these states molecules, atoms, or ions are in motion. The Difference Between Solids/Liquids/Gases On A Molecular Level.
From www.geeksforgeeks.org
Difference Between Solid, Liquid, and Gas In Tabular Form The Difference Between Solids/Liquids/Gases On A Molecular Level This model explains the higher density, greater order, and lower compressibility of liquids versus gases; This model explains the higher density, greater order, and lower compressibility of liquids versus gases; There are three different states of matter: In all these states molecules, atoms, or ions are in motion with a specific. Let us begin at the. Generally, the strength of. The Difference Between Solids/Liquids/Gases On A Molecular Level.
From www.youtube.com
CHEMISTRY 101 Compare the solid, liquid and gas phases at the The Difference Between Solids/Liquids/Gases On A Molecular Level The thermal expansion of liquids; What determines the phase of a substance? Generally, the strength of the. There are three different states of matter: This model explains the higher density, greater order, and lower compressibility of liquids versus gases; Figure \(\pageindex{2}\) shows the differences among solids, liquids, and gases at the molecular level. A solid has definite volume and shape,. The Difference Between Solids/Liquids/Gases On A Molecular Level.
From diagramlibraryconjoin.z19.web.core.windows.net
Solid Liquid And Gas Diagram The Difference Between Solids/Liquids/Gases On A Molecular Level The thermal expansion of liquids; There are three different states of matter: In all these states molecules, atoms, or ions are in motion with a specific. Generally, the strength of the. Gases, liquids and solids are all made up of atoms, molecules, and/or ions, but the behaviors of these particles differ in the three phases. What determines the phase of. The Difference Between Solids/Liquids/Gases On A Molecular Level.
From psiberg.com
Properties of Solid, Liquid, Gases A Comparison The Difference Between Solids/Liquids/Gases On A Molecular Level A solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. Let us begin at the. There are three different states of matter: The solid, the liquid, and the gas phase. Generally, the strength of the. This model explains the higher density, greater order,. The Difference Between Solids/Liquids/Gases On A Molecular Level.
From www.youtube.com
Difference between solid liquid and gas Molecules of solid liquid and The Difference Between Solids/Liquids/Gases On A Molecular Level What determines the phase of a substance? This model explains the higher density, greater order, and lower compressibility of liquids versus gases; The thermal expansion of liquids; Gases, liquids and solids are all made up of atoms, molecules, and/or ions, but the behaviors of these particles differ in the three phases. This model explains the higher density, greater order, and. The Difference Between Solids/Liquids/Gases On A Molecular Level.
From www.snexplores.org
Explainer What are the different states of matter? The Difference Between Solids/Liquids/Gases On A Molecular Level The solid, the liquid, and the gas phase. There are three different states of matter: In all these states molecules, atoms, or ions are in motion with a specific. Let us begin at the. Generally, the strength of the. The thermal expansion of liquids; This model explains the higher density, greater order, and lower compressibility of liquids versus gases; Gases,. The Difference Between Solids/Liquids/Gases On A Molecular Level.
From studylib.net
Chapter 11 Liquids and Solids A. Intermolecular Forces The Difference Between Solids/Liquids/Gases On A Molecular Level This model explains the higher density, greater order, and lower compressibility of liquids versus gases; In all these states molecules, atoms, or ions are in motion with a specific. Gases, liquids and solids are all made up of atoms, molecules, and/or ions, but the behaviors of these particles differ in the three phases. The thermal expansion of liquids; Figure \(\pageindex{2}\). The Difference Between Solids/Liquids/Gases On A Molecular Level.
From www.slideserve.com
PPT Particle model of solids, liquids and gases PowerPoint The Difference Between Solids/Liquids/Gases On A Molecular Level Generally, the strength of the. The thermal expansion of liquids; This model explains the higher density, greater order, and lower compressibility of liquids versus gases; Figure \(\pageindex{2}\) shows the differences among solids, liquids, and gases at the molecular level. This model explains the higher density, greater order, and lower compressibility of liquids versus gases; A solid has definite volume and. The Difference Between Solids/Liquids/Gases On A Molecular Level.
From guidelistbaquantising.z13.web.core.windows.net
Venn Diagram For Solids Liquids And Gases The Difference Between Solids/Liquids/Gases On A Molecular Level What determines the phase of a substance? This model explains the higher density, greater order, and lower compressibility of liquids versus gases; In all these states molecules, atoms, or ions are in motion with a specific. There are three different states of matter: Figure \(\pageindex{2}\) shows the differences among solids, liquids, and gases at the molecular level. Generally, the strength. The Difference Between Solids/Liquids/Gases On A Molecular Level.
From www.majordifferences.com
Difference between Solid, Liquid and Gas Table (Solids vs Liquids vs The Difference Between Solids/Liquids/Gases On A Molecular Level There are three different states of matter: What determines the phase of a substance? Generally, the strength of the. This model explains the higher density, greater order, and lower compressibility of liquids versus gases; Let us begin at the. This model explains the higher density, greater order, and lower compressibility of liquids versus gases; Figure \(\pageindex{2}\) shows the differences among. The Difference Between Solids/Liquids/Gases On A Molecular Level.
From www.ase.org.uk
Solids, liquids and gases The Difference Between Solids/Liquids/Gases On A Molecular Level Generally, the strength of the. Figure \(\pageindex{2}\) shows the differences among solids, liquids, and gases at the molecular level. This model explains the higher density, greater order, and lower compressibility of liquids versus gases; This model explains the higher density, greater order, and lower compressibility of liquids versus gases; Let us begin at the. What determines the phase of a. The Difference Between Solids/Liquids/Gases On A Molecular Level.
From www.theschoolrun.com
The difference between solids, liquids and gases TheSchoolRun The Difference Between Solids/Liquids/Gases On A Molecular Level In all these states molecules, atoms, or ions are in motion with a specific. Generally, the strength of the. This model explains the higher density, greater order, and lower compressibility of liquids versus gases; This model explains the higher density, greater order, and lower compressibility of liquids versus gases; The solid, the liquid, and the gas phase. A solid has. The Difference Between Solids/Liquids/Gases On A Molecular Level.
From socratic.org
What are examples of gases, liquids, and solids? Socratic The Difference Between Solids/Liquids/Gases On A Molecular Level In all these states molecules, atoms, or ions are in motion with a specific. Figure \(\pageindex{2}\) shows the differences among solids, liquids, and gases at the molecular level. Generally, the strength of the. The solid, the liquid, and the gas phase. This model explains the higher density, greater order, and lower compressibility of liquids versus gases; This model explains the. The Difference Between Solids/Liquids/Gases On A Molecular Level.
From www.learnatnoon.com
The Molecular Differences Between Solids, Liquid and Gases The Difference Between Solids/Liquids/Gases On A Molecular Level This model explains the higher density, greater order, and lower compressibility of liquids versus gases; In all these states molecules, atoms, or ions are in motion with a specific. A solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. Figure \(\pageindex{2}\) shows the. The Difference Between Solids/Liquids/Gases On A Molecular Level.