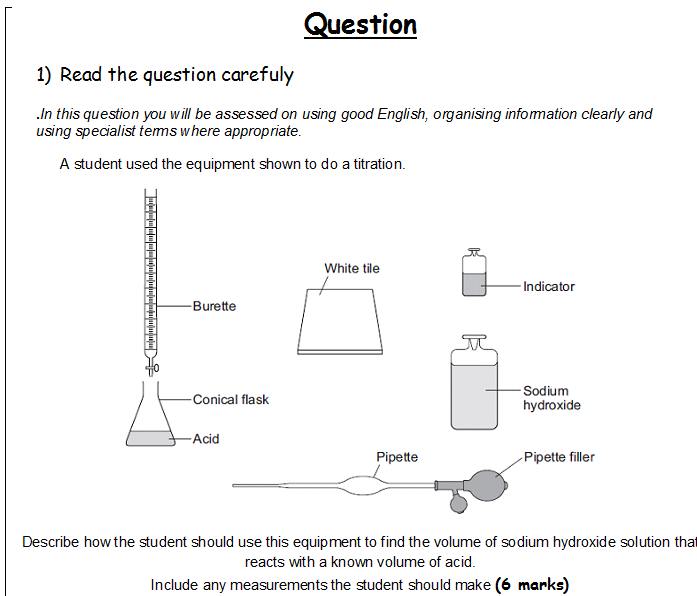
A Level Chemistry Titration Practical Questions . Moles in diluted sample = 0.003 mol (1) c = n/(v/1000) = 0.003/(25/1000) =. Determine the number of moles in the diluted sample of hydrochloric acid, and hence the concentration of the diluted acid. Use our revision notes to understand titration calculations for your a level chemistry course using worked examples. Titrate the contents of the conical flask by adding solution to it from the burette until the indicator undergoes a definite, permanent colour change. This experiment can be repeated by using a different volume of water that would result in a more accurate value for the enthalpy of.
from www.tes.com
Use our revision notes to understand titration calculations for your a level chemistry course using worked examples. This experiment can be repeated by using a different volume of water that would result in a more accurate value for the enthalpy of. Determine the number of moles in the diluted sample of hydrochloric acid, and hence the concentration of the diluted acid. Moles in diluted sample = 0.003 mol (1) c = n/(v/1000) = 0.003/(25/1000) =. Titrate the contents of the conical flask by adding solution to it from the burette until the indicator undergoes a definite, permanent colour change.
Structured 6 Mark Question Titration Exam Practice/ Exam Technique/ 6 Mark Help Teaching
A Level Chemistry Titration Practical Questions Moles in diluted sample = 0.003 mol (1) c = n/(v/1000) = 0.003/(25/1000) =. Titrate the contents of the conical flask by adding solution to it from the burette until the indicator undergoes a definite, permanent colour change. Determine the number of moles in the diluted sample of hydrochloric acid, and hence the concentration of the diluted acid. This experiment can be repeated by using a different volume of water that would result in a more accurate value for the enthalpy of. Moles in diluted sample = 0.003 mol (1) c = n/(v/1000) = 0.003/(25/1000) =. Use our revision notes to understand titration calculations for your a level chemistry course using worked examples.
From www.youtube.com
Redox Titration Example Question 2 (Easy) ALevel Chemistry YouTube A Level Chemistry Titration Practical Questions Moles in diluted sample = 0.003 mol (1) c = n/(v/1000) = 0.003/(25/1000) =. Use our revision notes to understand titration calculations for your a level chemistry course using worked examples. Determine the number of moles in the diluted sample of hydrochloric acid, and hence the concentration of the diluted acid. Titrate the contents of the conical flask by adding. A Level Chemistry Titration Practical Questions.
From www.stuvia.com
AQA ALevel Chemistry Mass Spectrometer, Titrations, Bonding Practice Exam Questions and Answers A Level Chemistry Titration Practical Questions This experiment can be repeated by using a different volume of water that would result in a more accurate value for the enthalpy of. Determine the number of moles in the diluted sample of hydrochloric acid, and hence the concentration of the diluted acid. Titrate the contents of the conical flask by adding solution to it from the burette until. A Level Chemistry Titration Practical Questions.
From www.scribd.com
Titration Questions PDF Titration Chemistry A Level Chemistry Titration Practical Questions Determine the number of moles in the diluted sample of hydrochloric acid, and hence the concentration of the diluted acid. Moles in diluted sample = 0.003 mol (1) c = n/(v/1000) = 0.003/(25/1000) =. Use our revision notes to understand titration calculations for your a level chemistry course using worked examples. This experiment can be repeated by using a different. A Level Chemistry Titration Practical Questions.
From studylib.net
Titration Practice Worksheet A Level Chemistry Titration Practical Questions Use our revision notes to understand titration calculations for your a level chemistry course using worked examples. Determine the number of moles in the diluted sample of hydrochloric acid, and hence the concentration of the diluted acid. Titrate the contents of the conical flask by adding solution to it from the burette until the indicator undergoes a definite, permanent colour. A Level Chemistry Titration Practical Questions.
From www.youtube.com
TITRATION EXAM QUESTION for A LEVEL CHEMISTRY YouTube A Level Chemistry Titration Practical Questions Moles in diluted sample = 0.003 mol (1) c = n/(v/1000) = 0.003/(25/1000) =. Titrate the contents of the conical flask by adding solution to it from the burette until the indicator undergoes a definite, permanent colour change. Determine the number of moles in the diluted sample of hydrochloric acid, and hence the concentration of the diluted acid. Use our. A Level Chemistry Titration Practical Questions.
From www.youtube.com
CHEMISTRY TITRATION PRACTICAL CALCULATION QUESTION AND SOLUTION jonahemmanuel titration YouTube A Level Chemistry Titration Practical Questions Determine the number of moles in the diluted sample of hydrochloric acid, and hence the concentration of the diluted acid. Titrate the contents of the conical flask by adding solution to it from the burette until the indicator undergoes a definite, permanent colour change. Use our revision notes to understand titration calculations for your a level chemistry course using worked. A Level Chemistry Titration Practical Questions.
From www.shalom-education.com
Required Practical Titration with a Strong Acid and a Strong Alkali GCSE Chemistry Revision A Level Chemistry Titration Practical Questions Moles in diluted sample = 0.003 mol (1) c = n/(v/1000) = 0.003/(25/1000) =. Determine the number of moles in the diluted sample of hydrochloric acid, and hence the concentration of the diluted acid. Use our revision notes to understand titration calculations for your a level chemistry course using worked examples. This experiment can be repeated by using a different. A Level Chemistry Titration Practical Questions.
From www.tes.com
A level Chemistry Titration Calculations Teaching Resources A Level Chemistry Titration Practical Questions Determine the number of moles in the diluted sample of hydrochloric acid, and hence the concentration of the diluted acid. Titrate the contents of the conical flask by adding solution to it from the burette until the indicator undergoes a definite, permanent colour change. Moles in diluted sample = 0.003 mol (1) c = n/(v/1000) = 0.003/(25/1000) =. Use our. A Level Chemistry Titration Practical Questions.
From www.studocu.com
Titration Exam Questions ACIDBASE TITRATIONS PAST EXAM QUESTIONS Section 1 Multiple Choice A Level Chemistry Titration Practical Questions Titrate the contents of the conical flask by adding solution to it from the burette until the indicator undergoes a definite, permanent colour change. This experiment can be repeated by using a different volume of water that would result in a more accurate value for the enthalpy of. Determine the number of moles in the diluted sample of hydrochloric acid,. A Level Chemistry Titration Practical Questions.
From lessonplans.ie
Mock titration chemistry questions ( booklet ) ( marking scheme included) Lesson Plans A Level Chemistry Titration Practical Questions This experiment can be repeated by using a different volume of water that would result in a more accurate value for the enthalpy of. Moles in diluted sample = 0.003 mol (1) c = n/(v/1000) = 0.003/(25/1000) =. Determine the number of moles in the diluted sample of hydrochloric acid, and hence the concentration of the diluted acid. Titrate the. A Level Chemistry Titration Practical Questions.
From www.revisely.co.uk
ALevel AQA Chemistry Questions pH Curves, Titrations and Indicators Revisely A Level Chemistry Titration Practical Questions Determine the number of moles in the diluted sample of hydrochloric acid, and hence the concentration of the diluted acid. Titrate the contents of the conical flask by adding solution to it from the burette until the indicator undergoes a definite, permanent colour change. Use our revision notes to understand titration calculations for your a level chemistry course using worked. A Level Chemistry Titration Practical Questions.
From www.youtube.com
solution to titration of phosphoric acid unit 5 aqa chemistry A2 level YouTube A Level Chemistry Titration Practical Questions Moles in diluted sample = 0.003 mol (1) c = n/(v/1000) = 0.003/(25/1000) =. Use our revision notes to understand titration calculations for your a level chemistry course using worked examples. This experiment can be repeated by using a different volume of water that would result in a more accurate value for the enthalpy of. Titrate the contents of the. A Level Chemistry Titration Practical Questions.
From www.tes.com
Titrations Required Practical Sheet Teaching Resources A Level Chemistry Titration Practical Questions Titrate the contents of the conical flask by adding solution to it from the burette until the indicator undergoes a definite, permanent colour change. Moles in diluted sample = 0.003 mol (1) c = n/(v/1000) = 0.003/(25/1000) =. Determine the number of moles in the diluted sample of hydrochloric acid, and hence the concentration of the diluted acid. This experiment. A Level Chemistry Titration Practical Questions.
From keplarllp.com
😂 Chemistry titration practical. Titration Tutorial Tips & Tricks for Titrating. 20190206 A Level Chemistry Titration Practical Questions Use our revision notes to understand titration calculations for your a level chemistry course using worked examples. Determine the number of moles in the diluted sample of hydrochloric acid, and hence the concentration of the diluted acid. Titrate the contents of the conical flask by adding solution to it from the burette until the indicator undergoes a definite, permanent colour. A Level Chemistry Titration Practical Questions.
From theedge.com.hk
Chemistry How To Titration The Edge A Level Chemistry Titration Practical Questions Use our revision notes to understand titration calculations for your a level chemistry course using worked examples. Titrate the contents of the conical flask by adding solution to it from the burette until the indicator undergoes a definite, permanent colour change. Moles in diluted sample = 0.003 mol (1) c = n/(v/1000) = 0.003/(25/1000) =. Determine the number of moles. A Level Chemistry Titration Practical Questions.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Foundation A Level Chemistry Titration Practical Questions Determine the number of moles in the diluted sample of hydrochloric acid, and hence the concentration of the diluted acid. Use our revision notes to understand titration calculations for your a level chemistry course using worked examples. This experiment can be repeated by using a different volume of water that would result in a more accurate value for the enthalpy. A Level Chemistry Titration Practical Questions.
From www.youtube.com
Titration Calculations GCSE Science grade 7, 8 and 9 Booster Questions YouTube A Level Chemistry Titration Practical Questions Moles in diluted sample = 0.003 mol (1) c = n/(v/1000) = 0.003/(25/1000) =. Use our revision notes to understand titration calculations for your a level chemistry course using worked examples. Titrate the contents of the conical flask by adding solution to it from the burette until the indicator undergoes a definite, permanent colour change. This experiment can be repeated. A Level Chemistry Titration Practical Questions.
From www.tes.com
FREE KS4 GCSE Chemistry (Science) Titration Calculation Questions Teaching Resources A Level Chemistry Titration Practical Questions This experiment can be repeated by using a different volume of water that would result in a more accurate value for the enthalpy of. Use our revision notes to understand titration calculations for your a level chemistry course using worked examples. Titrate the contents of the conical flask by adding solution to it from the burette until the indicator undergoes. A Level Chemistry Titration Practical Questions.
From www.savemyexams.com
Core Practical AcidAlkali Titration Edexcel GCSE Chemistry Revision Notes 2018 A Level Chemistry Titration Practical Questions Use our revision notes to understand titration calculations for your a level chemistry course using worked examples. This experiment can be repeated by using a different volume of water that would result in a more accurate value for the enthalpy of. Determine the number of moles in the diluted sample of hydrochloric acid, and hence the concentration of the diluted. A Level Chemistry Titration Practical Questions.
From chemistry.analia-sanchez.net
Titration Notes Chemistry Classes / Ronald Reagan S.H.S. A Level Chemistry Titration Practical Questions Moles in diluted sample = 0.003 mol (1) c = n/(v/1000) = 0.003/(25/1000) =. This experiment can be repeated by using a different volume of water that would result in a more accurate value for the enthalpy of. Titrate the contents of the conical flask by adding solution to it from the burette until the indicator undergoes a definite, permanent. A Level Chemistry Titration Practical Questions.
From www.tes.com
Structured 6 Mark Question Titration Exam Practice/ Exam Technique/ 6 Mark Help Teaching A Level Chemistry Titration Practical Questions This experiment can be repeated by using a different volume of water that would result in a more accurate value for the enthalpy of. Moles in diluted sample = 0.003 mol (1) c = n/(v/1000) = 0.003/(25/1000) =. Use our revision notes to understand titration calculations for your a level chemistry course using worked examples. Titrate the contents of the. A Level Chemistry Titration Practical Questions.
From www.youtube.com
An Introduction To Titration Calculations (GCSE Chemistry) YouTube A Level Chemistry Titration Practical Questions Use our revision notes to understand titration calculations for your a level chemistry course using worked examples. Moles in diluted sample = 0.003 mol (1) c = n/(v/1000) = 0.003/(25/1000) =. Determine the number of moles in the diluted sample of hydrochloric acid, and hence the concentration of the diluted acid. This experiment can be repeated by using a different. A Level Chemistry Titration Practical Questions.
From kaytrinasofya.blogspot.com
37+ titration calculation questions gcse A Level Chemistry Titration Practical Questions Determine the number of moles in the diluted sample of hydrochloric acid, and hence the concentration of the diluted acid. Use our revision notes to understand titration calculations for your a level chemistry course using worked examples. Moles in diluted sample = 0.003 mol (1) c = n/(v/1000) = 0.003/(25/1000) =. This experiment can be repeated by using a different. A Level Chemistry Titration Practical Questions.
From dokumen.tips
IGCSE Titration Practice Questions A Level Chemistry Titration Practical Questions Moles in diluted sample = 0.003 mol (1) c = n/(v/1000) = 0.003/(25/1000) =. Determine the number of moles in the diluted sample of hydrochloric acid, and hence the concentration of the diluted acid. Use our revision notes to understand titration calculations for your a level chemistry course using worked examples. This experiment can be repeated by using a different. A Level Chemistry Titration Practical Questions.
From www.scribd.com
AS Level Chemistry Practical Paper 3 Titration PDF Aldehyde Materials A Level Chemistry Titration Practical Questions This experiment can be repeated by using a different volume of water that would result in a more accurate value for the enthalpy of. Use our revision notes to understand titration calculations for your a level chemistry course using worked examples. Determine the number of moles in the diluted sample of hydrochloric acid, and hence the concentration of the diluted. A Level Chemistry Titration Practical Questions.
From www.tes.com
Chemistry Required Practical 2 Titration Teaching Resources A Level Chemistry Titration Practical Questions Determine the number of moles in the diluted sample of hydrochloric acid, and hence the concentration of the diluted acid. Use our revision notes to understand titration calculations for your a level chemistry course using worked examples. Moles in diluted sample = 0.003 mol (1) c = n/(v/1000) = 0.003/(25/1000) =. This experiment can be repeated by using a different. A Level Chemistry Titration Practical Questions.
From www.youtube.com
Acids and Bases Back Titration Calculation Exam Question|A Level Chemistry (AQA) YouTube A Level Chemistry Titration Practical Questions This experiment can be repeated by using a different volume of water that would result in a more accurate value for the enthalpy of. Determine the number of moles in the diluted sample of hydrochloric acid, and hence the concentration of the diluted acid. Use our revision notes to understand titration calculations for your a level chemistry course using worked. A Level Chemistry Titration Practical Questions.
From www.youtube.com
Redox Titrations A level Chemistry Question Walkthrough YouTube A Level Chemistry Titration Practical Questions Moles in diluted sample = 0.003 mol (1) c = n/(v/1000) = 0.003/(25/1000) =. This experiment can be repeated by using a different volume of water that would result in a more accurate value for the enthalpy of. Use our revision notes to understand titration calculations for your a level chemistry course using worked examples. Determine the number of moles. A Level Chemistry Titration Practical Questions.
From www.youtube.com
Titration How to perform Titration Experiment/Practical YouTube A Level Chemistry Titration Practical Questions Moles in diluted sample = 0.003 mol (1) c = n/(v/1000) = 0.003/(25/1000) =. This experiment can be repeated by using a different volume of water that would result in a more accurate value for the enthalpy of. Use our revision notes to understand titration calculations for your a level chemistry course using worked examples. Determine the number of moles. A Level Chemistry Titration Practical Questions.
From www.tes.com
Acids, titrations and redox OCR AS Chemistry Teaching Resources A Level Chemistry Titration Practical Questions This experiment can be repeated by using a different volume of water that would result in a more accurate value for the enthalpy of. Titrate the contents of the conical flask by adding solution to it from the burette until the indicator undergoes a definite, permanent colour change. Determine the number of moles in the diluted sample of hydrochloric acid,. A Level Chemistry Titration Practical Questions.
From www.scribd.com
IGCSE Titration Practice Questions A Level Chemistry Titration Practical Questions Moles in diluted sample = 0.003 mol (1) c = n/(v/1000) = 0.003/(25/1000) =. Use our revision notes to understand titration calculations for your a level chemistry course using worked examples. Titrate the contents of the conical flask by adding solution to it from the burette until the indicator undergoes a definite, permanent colour change. This experiment can be repeated. A Level Chemistry Titration Practical Questions.
From www.scribd.com
GCSE Chemistry Titrations Questions With Answers PDF Chemistry Titration A Level Chemistry Titration Practical Questions Titrate the contents of the conical flask by adding solution to it from the burette until the indicator undergoes a definite, permanent colour change. Moles in diluted sample = 0.003 mol (1) c = n/(v/1000) = 0.003/(25/1000) =. Use our revision notes to understand titration calculations for your a level chemistry course using worked examples. This experiment can be repeated. A Level Chemistry Titration Practical Questions.
From www.thinkswap.com
Titration Practical Chemistry Year 12 SACE Thinkswap A Level Chemistry Titration Practical Questions Titrate the contents of the conical flask by adding solution to it from the burette until the indicator undergoes a definite, permanent colour change. Use our revision notes to understand titration calculations for your a level chemistry course using worked examples. Determine the number of moles in the diluted sample of hydrochloric acid, and hence the concentration of the diluted. A Level Chemistry Titration Practical Questions.
From www.thinkswap.com
Titration Practical Chemistry Year 12 SACE Thinkswap A Level Chemistry Titration Practical Questions Use our revision notes to understand titration calculations for your a level chemistry course using worked examples. Titrate the contents of the conical flask by adding solution to it from the burette until the indicator undergoes a definite, permanent colour change. This experiment can be repeated by using a different volume of water that would result in a more accurate. A Level Chemistry Titration Practical Questions.
From thescienceteacher.co.uk
Concentration and titrations teaching resources the science teacher A Level Chemistry Titration Practical Questions This experiment can be repeated by using a different volume of water that would result in a more accurate value for the enthalpy of. Use our revision notes to understand titration calculations for your a level chemistry course using worked examples. Determine the number of moles in the diluted sample of hydrochloric acid, and hence the concentration of the diluted. A Level Chemistry Titration Practical Questions.