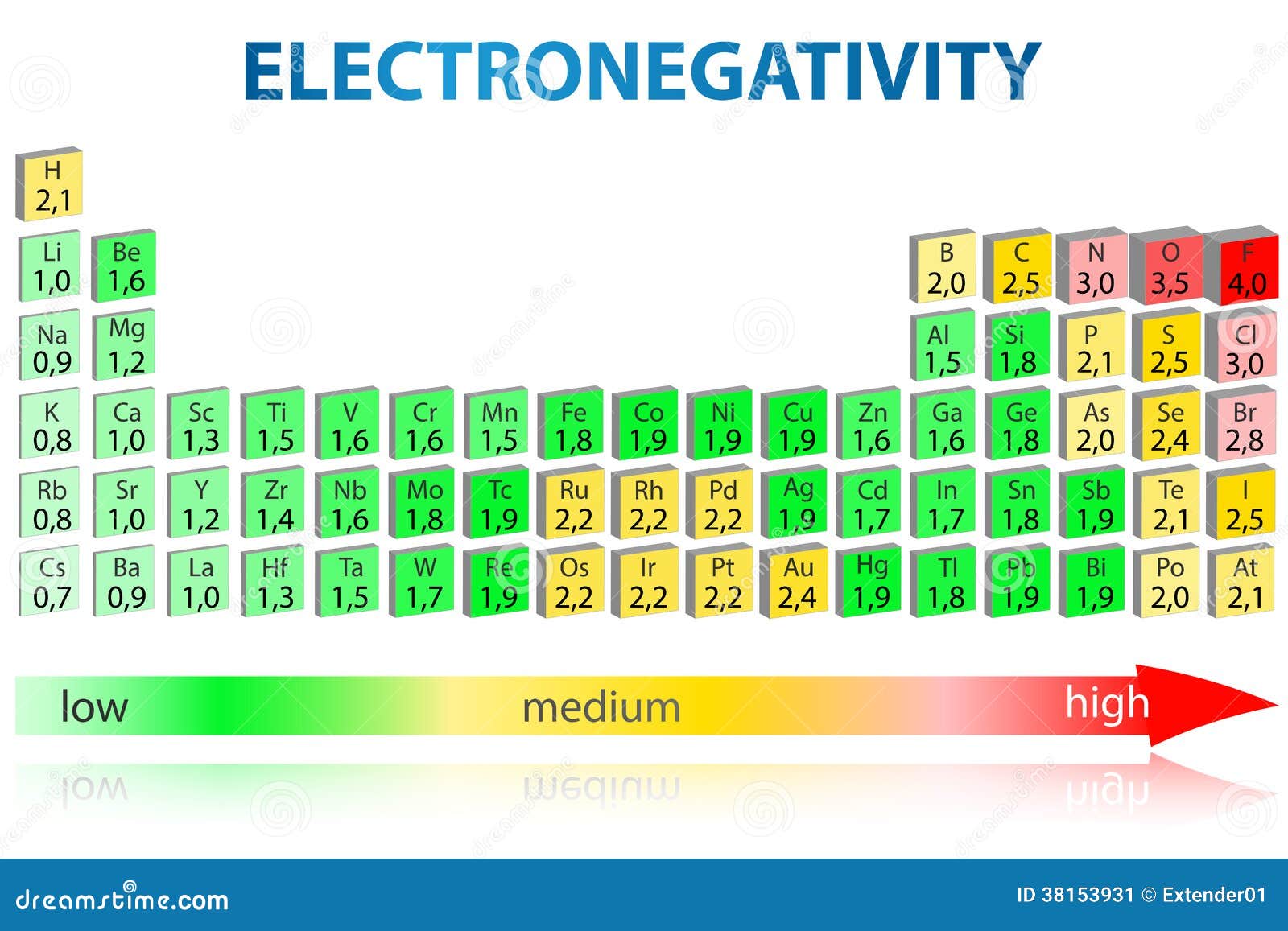
Properties Of Halogen Electronegativity . Fluorine is the most electronegative element in the periodic table. Electronegativity is the tendency of an atom to attract a shared pair of electrons in a covalent bond towards itself. We will cover electronegativity, boiling point, and solubility of silver halides. Fluorine has the highest electronegativity of all elements. Halogens are elements found in group 7 of the periodic table. The electronegativity of an atom refers to how strongly it attracts electrons towards itself in a covalent bond; The halogens are particularly reactive with the alkali metals and alkaline earths, forming stable ionic crystals. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. It is usually measured on the pauling scale, on which the most electronegative element. In this section, we will explore some core trends in the properties of the halogens. It appears as a pale yellow gas at room temperature.
from www.dreamstime.com
Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The electronegativity of an atom refers to how strongly it attracts electrons towards itself in a covalent bond; The halogens are particularly reactive with the alkali metals and alkaline earths, forming stable ionic crystals. Electronegativity is the tendency of an atom to attract a shared pair of electrons in a covalent bond towards itself. We will cover electronegativity, boiling point, and solubility of silver halides. Fluorine has the highest electronegativity of all elements. In this section, we will explore some core trends in the properties of the halogens. It is usually measured on the pauling scale, on which the most electronegative element. Halogens are elements found in group 7 of the periodic table. It appears as a pale yellow gas at room temperature.
Electronegativity Periodic Table Stock Illustration Illustration of trend, properties 38153931
Properties Of Halogen Electronegativity Fluorine has the highest electronegativity of all elements. Fluorine has the highest electronegativity of all elements. It appears as a pale yellow gas at room temperature. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. We will cover electronegativity, boiling point, and solubility of silver halides. The halogens are particularly reactive with the alkali metals and alkaline earths, forming stable ionic crystals. Halogens are elements found in group 7 of the periodic table. Electronegativity is the tendency of an atom to attract a shared pair of electrons in a covalent bond towards itself. It is usually measured on the pauling scale, on which the most electronegative element. The electronegativity of an atom refers to how strongly it attracts electrons towards itself in a covalent bond; Fluorine is the most electronegative element in the periodic table. In this section, we will explore some core trends in the properties of the halogens.
From www.slideserve.com
PPT The Halogens PowerPoint Presentation, free download ID5949337 Properties Of Halogen Electronegativity It appears as a pale yellow gas at room temperature. It is usually measured on the pauling scale, on which the most electronegative element. The electronegativity of an atom refers to how strongly it attracts electrons towards itself in a covalent bond; Fluorine has the highest electronegativity of all elements. Electronegativity is a measure of the tendency of an atom. Properties Of Halogen Electronegativity.
From www.slideserve.com
PPT Characteristic Properties of the Halogens PowerPoint Presentation ID370141 Properties Of Halogen Electronegativity Halogens are elements found in group 7 of the periodic table. Fluorine is the most electronegative element in the periodic table. It is usually measured on the pauling scale, on which the most electronegative element. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. Electronegativity is the tendency of an atom to. Properties Of Halogen Electronegativity.
From collegedunia.com
Halogens Properties, Electronic Configuration & Characteristics Properties Of Halogen Electronegativity The halogens are particularly reactive with the alkali metals and alkaline earths, forming stable ionic crystals. It is usually measured on the pauling scale, on which the most electronegative element. Fluorine has the highest electronegativity of all elements. The electronegativity of an atom refers to how strongly it attracts electrons towards itself in a covalent bond; We will cover electronegativity,. Properties Of Halogen Electronegativity.
From ar.inspiredpencil.com
Electronegativity Diagram Properties Of Halogen Electronegativity In this section, we will explore some core trends in the properties of the halogens. Electronegativity is the tendency of an atom to attract a shared pair of electrons in a covalent bond towards itself. It is usually measured on the pauling scale, on which the most electronegative element. Halogens are elements found in group 7 of the periodic table.. Properties Of Halogen Electronegativity.
From www.chemistrystudent.com
Group 7 Halogens and their Electronegativities (ALevel) ChemistryStudent Properties Of Halogen Electronegativity It is usually measured on the pauling scale, on which the most electronegative element. Halogens are elements found in group 7 of the periodic table. Fluorine has the highest electronegativity of all elements. In this section, we will explore some core trends in the properties of the halogens. It appears as a pale yellow gas at room temperature. The halogens. Properties Of Halogen Electronegativity.
From slideplayer.com
The Periodic Table. ppt download Properties Of Halogen Electronegativity It appears as a pale yellow gas at room temperature. Electronegativity is the tendency of an atom to attract a shared pair of electrons in a covalent bond towards itself. We will cover electronegativity, boiling point, and solubility of silver halides. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. Fluorine has. Properties Of Halogen Electronegativity.
From www.slideserve.com
PPT Chapter 22 Chemistry of the Nonmetals PowerPoint Presentation, free download ID6088597 Properties Of Halogen Electronegativity Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. Electronegativity is the tendency of an atom to attract a shared pair of electrons in a covalent bond towards itself. We will cover electronegativity, boiling point, and solubility of silver halides. Halogens are elements found in group 7 of the periodic table. Fluorine. Properties Of Halogen Electronegativity.
From iperiodictable.com
What is Electronegativity Chart List of Electronegativity [PDF] Periodic Table Properties Of Halogen Electronegativity We will cover electronegativity, boiling point, and solubility of silver halides. Electronegativity is the tendency of an atom to attract a shared pair of electrons in a covalent bond towards itself. Halogens are elements found in group 7 of the periodic table. The halogens are particularly reactive with the alkali metals and alkaline earths, forming stable ionic crystals. Fluorine has. Properties Of Halogen Electronegativity.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID6702617 Properties Of Halogen Electronegativity We will cover electronegativity, boiling point, and solubility of silver halides. Fluorine is the most electronegative element in the periodic table. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. Fluorine has the highest electronegativity of all elements. Halogens are elements found in group 7 of the periodic table. It appears as. Properties Of Halogen Electronegativity.
From chemizi.blogspot.com
Halogen elementsdefinitionpropertiesreactivity and uses. Properties Of Halogen Electronegativity The electronegativity of an atom refers to how strongly it attracts electrons towards itself in a covalent bond; It is usually measured on the pauling scale, on which the most electronegative element. Fluorine is the most electronegative element in the periodic table. Fluorine has the highest electronegativity of all elements. Electronegativity is the tendency of an atom to attract a. Properties Of Halogen Electronegativity.
From www.vedantu.com
Halogens Learn Definition, Properties, Facts & Examples Properties Of Halogen Electronegativity We will cover electronegativity, boiling point, and solubility of silver halides. It appears as a pale yellow gas at room temperature. Electronegativity is the tendency of an atom to attract a shared pair of electrons in a covalent bond towards itself. The halogens are particularly reactive with the alkali metals and alkaline earths, forming stable ionic crystals. In this section,. Properties Of Halogen Electronegativity.
From www.thoughtco.com
Halogen Elements and Properties Properties Of Halogen Electronegativity Electronegativity is the tendency of an atom to attract a shared pair of electrons in a covalent bond towards itself. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The electronegativity of an atom refers to how strongly it attracts electrons towards itself in a covalent bond; In this section, we will. Properties Of Halogen Electronegativity.
From www.chemistrylearner.com
Electronegativity Definition, Value Chart, and Trend in Periodic Table Properties Of Halogen Electronegativity Electronegativity is the tendency of an atom to attract a shared pair of electrons in a covalent bond towards itself. The electronegativity of an atom refers to how strongly it attracts electrons towards itself in a covalent bond; We will cover electronegativity, boiling point, and solubility of silver halides. Fluorine is the most electronegative element in the periodic table. The. Properties Of Halogen Electronegativity.
From www.researchgate.net
Some properties of the Halogens Download Table Properties Of Halogen Electronegativity Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. In this section, we will explore some core trends in the properties of the halogens. We will cover electronegativity, boiling point, and solubility of silver halides. Halogens are elements found in group 7 of the periodic table. It is usually measured on the. Properties Of Halogen Electronegativity.
From scienceinfo.com
Chemical Properties of Halogen Elements and Hydrogen Halides Properties Of Halogen Electronegativity The halogens are particularly reactive with the alkali metals and alkaline earths, forming stable ionic crystals. We will cover electronegativity, boiling point, and solubility of silver halides. It appears as a pale yellow gas at room temperature. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. Electronegativity is the tendency of an. Properties Of Halogen Electronegativity.
From www.pinterest.com
Halogen Elements List and Facts Electron configuration, Electron affinity, Nuclear medicine Properties Of Halogen Electronegativity Halogens are elements found in group 7 of the periodic table. We will cover electronegativity, boiling point, and solubility of silver halides. The halogens are particularly reactive with the alkali metals and alkaline earths, forming stable ionic crystals. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. In this section, we will. Properties Of Halogen Electronegativity.
From www.slideserve.com
PPT The Halogens PowerPoint Presentation, free download ID1024240 Properties Of Halogen Electronegativity Fluorine is the most electronegative element in the periodic table. It is usually measured on the pauling scale, on which the most electronegative element. It appears as a pale yellow gas at room temperature. Fluorine has the highest electronegativity of all elements. Halogens are elements found in group 7 of the periodic table. Electronegativity is the tendency of an atom. Properties Of Halogen Electronegativity.
From sciencenotes.org
Electronegativity Definition and Trend Properties Of Halogen Electronegativity Electronegativity is the tendency of an atom to attract a shared pair of electrons in a covalent bond towards itself. We will cover electronegativity, boiling point, and solubility of silver halides. The electronegativity of an atom refers to how strongly it attracts electrons towards itself in a covalent bond; Halogens are elements found in group 7 of the periodic table.. Properties Of Halogen Electronegativity.
From www.dreamstime.com
Electronegativity Periodic Table Stock Photo Image of elements, electrons 37855044 Properties Of Halogen Electronegativity The electronegativity of an atom refers to how strongly it attracts electrons towards itself in a covalent bond; It appears as a pale yellow gas at room temperature. Halogens are elements found in group 7 of the periodic table. It is usually measured on the pauling scale, on which the most electronegative element. In this section, we will explore some. Properties Of Halogen Electronegativity.
From www.slideserve.com
PPT Halogens PowerPoint Presentation ID2110165 Properties Of Halogen Electronegativity The halogens are particularly reactive with the alkali metals and alkaline earths, forming stable ionic crystals. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. We will cover electronegativity, boiling point, and solubility of silver halides. Halogens are elements found in group 7 of the periodic table. Fluorine has the highest electronegativity. Properties Of Halogen Electronegativity.
From chemistry.com.pk
Electronegativity and Electronegativity Chart in PDF Properties Of Halogen Electronegativity Fluorine is the most electronegative element in the periodic table. The electronegativity of an atom refers to how strongly it attracts electrons towards itself in a covalent bond; We will cover electronegativity, boiling point, and solubility of silver halides. Fluorine has the highest electronegativity of all elements. Electronegativity is a measure of the tendency of an atom to attract a. Properties Of Halogen Electronegativity.
From www.slideserve.com
PPT Characteristic Properties of the Halogens PowerPoint Presentation ID370141 Properties Of Halogen Electronegativity The electronegativity of an atom refers to how strongly it attracts electrons towards itself in a covalent bond; We will cover electronegativity, boiling point, and solubility of silver halides. In this section, we will explore some core trends in the properties of the halogens. The halogens are particularly reactive with the alkali metals and alkaline earths, forming stable ionic crystals.. Properties Of Halogen Electronegativity.
From anthonystrendsassignment.weebly.com
Electronegativity Periodic Trends Properties Of Halogen Electronegativity Fluorine is the most electronegative element in the periodic table. In this section, we will explore some core trends in the properties of the halogens. Fluorine has the highest electronegativity of all elements. It appears as a pale yellow gas at room temperature. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons.. Properties Of Halogen Electronegativity.
From www.slideserve.com
PPT Characteristic Properties of the Halogens PowerPoint Presentation ID370141 Properties Of Halogen Electronegativity We will cover electronegativity, boiling point, and solubility of silver halides. Halogens are elements found in group 7 of the periodic table. Electronegativity is the tendency of an atom to attract a shared pair of electrons in a covalent bond towards itself. The electronegativity of an atom refers to how strongly it attracts electrons towards itself in a covalent bond;. Properties Of Halogen Electronegativity.
From www.dreamstime.com
Electronegativity Periodic Table Stock Illustration Illustration of trend, properties 38153931 Properties Of Halogen Electronegativity The electronegativity of an atom refers to how strongly it attracts electrons towards itself in a covalent bond; It is usually measured on the pauling scale, on which the most electronegative element. It appears as a pale yellow gas at room temperature. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. In. Properties Of Halogen Electronegativity.
From www.learnatnoon.com
Which Halogen Has The Lowest Electronegativity? Noon Academy Properties Of Halogen Electronegativity Fluorine has the highest electronegativity of all elements. Electronegativity is the tendency of an atom to attract a shared pair of electrons in a covalent bond towards itself. It appears as a pale yellow gas at room temperature. Fluorine is the most electronegative element in the periodic table. We will cover electronegativity, boiling point, and solubility of silver halides. The. Properties Of Halogen Electronegativity.
From mavink.com
Element Electronegativity Chart Properties Of Halogen Electronegativity The electronegativity of an atom refers to how strongly it attracts electrons towards itself in a covalent bond; Halogens are elements found in group 7 of the periodic table. We will cover electronegativity, boiling point, and solubility of silver halides. It is usually measured on the pauling scale, on which the most electronegative element. In this section, we will explore. Properties Of Halogen Electronegativity.
From exotfbtmr.blob.core.windows.net
Chemical Properties Of Halogen Group at Edward Cunningham blog Properties Of Halogen Electronegativity We will cover electronegativity, boiling point, and solubility of silver halides. The electronegativity of an atom refers to how strongly it attracts electrons towards itself in a covalent bond; Fluorine has the highest electronegativity of all elements. Fluorine is the most electronegative element in the periodic table. Halogens are elements found in group 7 of the periodic table. Electronegativity is. Properties Of Halogen Electronegativity.
From www.slideserve.com
PPT Characteristic Properties of the Halogens PowerPoint Presentation ID370141 Properties Of Halogen Electronegativity It is usually measured on the pauling scale, on which the most electronegative element. The electronegativity of an atom refers to how strongly it attracts electrons towards itself in a covalent bond; Halogens are elements found in group 7 of the periodic table. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons.. Properties Of Halogen Electronegativity.
From gampmagazine.weebly.com
Halogen periodic table gampmagazine Properties Of Halogen Electronegativity Halogens are elements found in group 7 of the periodic table. We will cover electronegativity, boiling point, and solubility of silver halides. It appears as a pale yellow gas at room temperature. Fluorine has the highest electronegativity of all elements. The halogens are particularly reactive with the alkali metals and alkaline earths, forming stable ionic crystals. The electronegativity of an. Properties Of Halogen Electronegativity.
From www.savemyexams.com
Chemical Properties of the Halogens & Hydrogen Halides CIE A Level Chemistry Revision Notes 2025 Properties Of Halogen Electronegativity Electronegativity is the tendency of an atom to attract a shared pair of electrons in a covalent bond towards itself. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. We will cover electronegativity, boiling point, and solubility of silver halides. The electronegativity of an atom refers to how strongly it attracts electrons. Properties Of Halogen Electronegativity.
From chemisfast.blogspot.com
Halogen family elementspropertiesperiodic tableoxyacidsradioactivity. PG.CHEMEASY Properties Of Halogen Electronegativity Fluorine has the highest electronegativity of all elements. It appears as a pale yellow gas at room temperature. In this section, we will explore some core trends in the properties of the halogens. We will cover electronegativity, boiling point, and solubility of silver halides. Halogens are elements found in group 7 of the periodic table. It is usually measured on. Properties Of Halogen Electronegativity.
From www.chemistrystudent.com
Group 7 Halogens and their Electronegativities (ALevel) ChemistryStudent Properties Of Halogen Electronegativity In this section, we will explore some core trends in the properties of the halogens. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. Fluorine has the highest electronegativity of all elements. The halogens are particularly reactive with the alkali metals and alkaline earths, forming stable ionic crystals. We will cover electronegativity,. Properties Of Halogen Electronegativity.
From www.numerade.com
SOLVED The following chart shows the electronegativity of four halogens Halogens Properties Of Halogen Electronegativity The electronegativity of an atom refers to how strongly it attracts electrons towards itself in a covalent bond; Fluorine has the highest electronegativity of all elements. It appears as a pale yellow gas at room temperature. The halogens are particularly reactive with the alkali metals and alkaline earths, forming stable ionic crystals. It is usually measured on the pauling scale,. Properties Of Halogen Electronegativity.
From www.slideserve.com
PPT The Halogens PowerPoint Presentation, free download ID2972542 Properties Of Halogen Electronegativity It is usually measured on the pauling scale, on which the most electronegative element. Electronegativity is the tendency of an atom to attract a shared pair of electrons in a covalent bond towards itself. In this section, we will explore some core trends in the properties of the halogens. We will cover electronegativity, boiling point, and solubility of silver halides.. Properties Of Halogen Electronegativity.