Volumetric Analysis Acid-Base Titration Lab Report . For our experiment, the titrant will be a solution of potassium hydrogen phalate (khp) which is behaving as an acid. This will be a standard. The titrant is the substance in which we know the amount of moles. In part b the concentration. A titration is a technique, in which a reagent, called a titrant, of known concentration is. In part a, the concentration of naoh found in the first titration is 1/l and in the second titration the titration is 2/l. The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium hydroxide, a strong base and acetic acid, a weak acid with sodium hydroxide,. Volume measurements play a key role in. Those two concentrations are closed to one another but are not exact. The goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via volumetric analysis, making use of the reaction of acetic acid with a strong base,. Titration is a common laboratory method of quantitative/chemical analysis that can be used to determine the concentration of a known reactant.
from www.chegg.com
This will be a standard. The titrant is the substance in which we know the amount of moles. Titration is a common laboratory method of quantitative/chemical analysis that can be used to determine the concentration of a known reactant. Those two concentrations are closed to one another but are not exact. In part a, the concentration of naoh found in the first titration is 1/l and in the second titration the titration is 2/l. The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium hydroxide, a strong base and acetic acid, a weak acid with sodium hydroxide,. The goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via volumetric analysis, making use of the reaction of acetic acid with a strong base,. In part b the concentration. Volume measurements play a key role in. A titration is a technique, in which a reagent, called a titrant, of known concentration is.
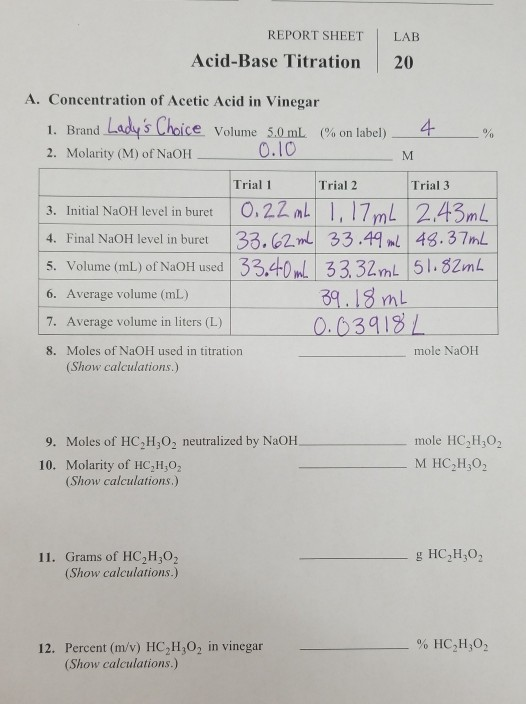
Solved REPORT SHEET LAB AcidBase Titration 20 A.
Volumetric Analysis Acid-Base Titration Lab Report Volume measurements play a key role in. In part a, the concentration of naoh found in the first titration is 1/l and in the second titration the titration is 2/l. The goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via volumetric analysis, making use of the reaction of acetic acid with a strong base,. In part b the concentration. Titration is a common laboratory method of quantitative/chemical analysis that can be used to determine the concentration of a known reactant. Volume measurements play a key role in. The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium hydroxide, a strong base and acetic acid, a weak acid with sodium hydroxide,. The titrant is the substance in which we know the amount of moles. This will be a standard. Those two concentrations are closed to one another but are not exact. A titration is a technique, in which a reagent, called a titrant, of known concentration is. For our experiment, the titrant will be a solution of potassium hydrogen phalate (khp) which is behaving as an acid.
From www.chegg.com
Volumetric Analysis AcidBase Titration Lab Report. Volumetric Analysis Acid-Base Titration Lab Report The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium hydroxide, a strong base and acetic acid, a weak acid with sodium hydroxide,. In part a, the concentration of naoh found in the first titration is 1/l and in the second titration the titration is 2/l. The goal of this experiment is. Volumetric Analysis Acid-Base Titration Lab Report.
From studylib.net
Exp 13 Volumetric Analysis AcidBase titration Titration Volumetric Analysis Acid-Base Titration Lab Report For our experiment, the titrant will be a solution of potassium hydrogen phalate (khp) which is behaving as an acid. Titration is a common laboratory method of quantitative/chemical analysis that can be used to determine the concentration of a known reactant. The titrant is the substance in which we know the amount of moles. In part a, the concentration of. Volumetric Analysis Acid-Base Titration Lab Report.
From mavink.com
Acid Base Titration Lab Procedure Volumetric Analysis Acid-Base Titration Lab Report The titrant is the substance in which we know the amount of moles. Titration is a common laboratory method of quantitative/chemical analysis that can be used to determine the concentration of a known reactant. This will be a standard. In part b the concentration. Those two concentrations are closed to one another but are not exact. The goal of this. Volumetric Analysis Acid-Base Titration Lab Report.
From www.thinkswap.com
Complete Acid/Base Titration Report Chemistry Year 12 HSC Thinkswap Volumetric Analysis Acid-Base Titration Lab Report The titrant is the substance in which we know the amount of moles. For our experiment, the titrant will be a solution of potassium hydrogen phalate (khp) which is behaving as an acid. The goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via volumetric analysis, making use of the reaction of acetic acid. Volumetric Analysis Acid-Base Titration Lab Report.
From www.studypool.com
SOLUTION Practicle 04 volumetric analysis acid base titration Studypool Volumetric Analysis Acid-Base Titration Lab Report The goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via volumetric analysis, making use of the reaction of acetic acid with a strong base,. For our experiment, the titrant will be a solution of potassium hydrogen phalate (khp) which is behaving as an acid. Titration is a common laboratory method of quantitative/chemical analysis. Volumetric Analysis Acid-Base Titration Lab Report.
From exymuypdr.blob.core.windows.net
Titration Acid Base Experiment at Robert Prescott blog Volumetric Analysis Acid-Base Titration Lab Report Those two concentrations are closed to one another but are not exact. Titration is a common laboratory method of quantitative/chemical analysis that can be used to determine the concentration of a known reactant. This will be a standard. Volume measurements play a key role in. For our experiment, the titrant will be a solution of potassium hydrogen phalate (khp) which. Volumetric Analysis Acid-Base Titration Lab Report.
From www.youtube.com
Acid base titrationVolumetric analysispharmaceutical chemistry Volumetric Analysis Acid-Base Titration Lab Report The goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via volumetric analysis, making use of the reaction of acetic acid with a strong base,. Titration is a common laboratory method of quantitative/chemical analysis that can be used to determine the concentration of a known reactant. For our experiment, the titrant will be a. Volumetric Analysis Acid-Base Titration Lab Report.
From mungfali.com
Acid Base Titration Calculation Volumetric Analysis Acid-Base Titration Lab Report The titrant is the substance in which we know the amount of moles. Volume measurements play a key role in. For our experiment, the titrant will be a solution of potassium hydrogen phalate (khp) which is behaving as an acid. In part a, the concentration of naoh found in the first titration is 1/l and in the second titration the. Volumetric Analysis Acid-Base Titration Lab Report.
From www.docsity.com
Acid Base Titrations Lab Report General Chemistry Lab CHEM 1045 Volumetric Analysis Acid-Base Titration Lab Report The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium hydroxide, a strong base and acetic acid, a weak acid with sodium hydroxide,. The goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via volumetric analysis, making use of the reaction of acetic acid with a. Volumetric Analysis Acid-Base Titration Lab Report.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Volumetric Analysis Acid-Base Titration Lab Report This will be a standard. A titration is a technique, in which a reagent, called a titrant, of known concentration is. The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium hydroxide, a strong base and acetic acid, a weak acid with sodium hydroxide,. Volume measurements play a key role in. The. Volumetric Analysis Acid-Base Titration Lab Report.
From www.chegg.com
Solved volumetric analysis titrations with acids and bases Volumetric Analysis Acid-Base Titration Lab Report In part a, the concentration of naoh found in the first titration is 1/l and in the second titration the titration is 2/l. For our experiment, the titrant will be a solution of potassium hydrogen phalate (khp) which is behaving as an acid. In part b the concentration. Those two concentrations are closed to one another but are not exact.. Volumetric Analysis Acid-Base Titration Lab Report.
From www.numerade.com
SOLVED CHEM 101 GENERAL CHEMISTRY Lab 7 AcidBase Titration (A Volumetric Analysis Acid-Base Titration Lab Report In part b the concentration. In part a, the concentration of naoh found in the first titration is 1/l and in the second titration the titration is 2/l. Those two concentrations are closed to one another but are not exact. Titration is a common laboratory method of quantitative/chemical analysis that can be used to determine the concentration of a known. Volumetric Analysis Acid-Base Titration Lab Report.
From modernalternativemama.com
Acid Base Titration Experiment Report Volumetric Analysis Acid-Base Titration Lab Report A titration is a technique, in which a reagent, called a titrant, of known concentration is. Volume measurements play a key role in. The goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via volumetric analysis, making use of the reaction of acetic acid with a strong base,. Those two concentrations are closed to. Volumetric Analysis Acid-Base Titration Lab Report.
From www.scribd.com
9.2 Volumetric Analysis.pptx.pdf Titration Atoms Volumetric Analysis Acid-Base Titration Lab Report The goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via volumetric analysis, making use of the reaction of acetic acid with a strong base,. The titrant is the substance in which we know the amount of moles. Volume measurements play a key role in. In part a, the concentration of naoh found in. Volumetric Analysis Acid-Base Titration Lab Report.
From www.chegg.com
Solved REPORT SHEET LAB AcidBase Titration 20 A. Volumetric Analysis Acid-Base Titration Lab Report The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium hydroxide, a strong base and acetic acid, a weak acid with sodium hydroxide,. The goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via volumetric analysis, making use of the reaction of acetic acid with a. Volumetric Analysis Acid-Base Titration Lab Report.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Volumetric Analysis Acid-Base Titration Lab Report The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium hydroxide, a strong base and acetic acid, a weak acid with sodium hydroxide,. The titrant is the substance in which we know the amount of moles. Titration is a common laboratory method of quantitative/chemical analysis that can be used to determine the. Volumetric Analysis Acid-Base Titration Lab Report.
From www.studypool.com
SOLUTION Acid base titration objective to determine the concentration Volumetric Analysis Acid-Base Titration Lab Report Volume measurements play a key role in. In part b the concentration. This will be a standard. The titrant is the substance in which we know the amount of moles. In part a, the concentration of naoh found in the first titration is 1/l and in the second titration the titration is 2/l. The goal of this experiment is to. Volumetric Analysis Acid-Base Titration Lab Report.
From www.chegg.com
Solved Section Titration Lab Report Sheet Table 1 Acid Base Volumetric Analysis Acid-Base Titration Lab Report In part a, the concentration of naoh found in the first titration is 1/l and in the second titration the titration is 2/l. The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium hydroxide, a strong base and acetic acid, a weak acid with sodium hydroxide,. A titration is a technique, in. Volumetric Analysis Acid-Base Titration Lab Report.
From cindy-bogspotacosta.blogspot.com
Acid Base Titration Experiment Report Volumetric Analysis Acid-Base Titration Lab Report For our experiment, the titrant will be a solution of potassium hydrogen phalate (khp) which is behaving as an acid. In part a, the concentration of naoh found in the first titration is 1/l and in the second titration the titration is 2/l. Titration is a common laboratory method of quantitative/chemical analysis that can be used to determine the concentration. Volumetric Analysis Acid-Base Titration Lab Report.
From www.poshpooch.ca
Acid base titrations lab report. 24/7 College Homework Help. Volumetric Analysis Acid-Base Titration Lab Report A titration is a technique, in which a reagent, called a titrant, of known concentration is. Volume measurements play a key role in. In part b the concentration. Those two concentrations are closed to one another but are not exact. The titrant is the substance in which we know the amount of moles. The purpose of this experiment is to. Volumetric Analysis Acid-Base Titration Lab Report.
From www.chegg.com
Solved Volumetric Analysis AcidBase Titration Data Sheet Volumetric Analysis Acid-Base Titration Lab Report Titration is a common laboratory method of quantitative/chemical analysis that can be used to determine the concentration of a known reactant. Volume measurements play a key role in. The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium hydroxide, a strong base and acetic acid, a weak acid with sodium hydroxide,. A. Volumetric Analysis Acid-Base Titration Lab Report.
From www.chegg.com
Solved Data Sheet Volumetric Analysis AcidBase Titration Volumetric Analysis Acid-Base Titration Lab Report For our experiment, the titrant will be a solution of potassium hydrogen phalate (khp) which is behaving as an acid. The titrant is the substance in which we know the amount of moles. This will be a standard. A titration is a technique, in which a reagent, called a titrant, of known concentration is. In part b the concentration. The. Volumetric Analysis Acid-Base Titration Lab Report.
From www.studypool.com
SOLUTION Practicle 04 volumetric analysis acid base titration Studypool Volumetric Analysis Acid-Base Titration Lab Report In part b the concentration. This will be a standard. The titrant is the substance in which we know the amount of moles. The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium hydroxide, a strong base and acetic acid, a weak acid with sodium hydroxide,. In part a, the concentration of. Volumetric Analysis Acid-Base Titration Lab Report.
From haileeqohicks.blogspot.com
Acid Base Titration Experiment Report HaileeqoHicks Volumetric Analysis Acid-Base Titration Lab Report Titration is a common laboratory method of quantitative/chemical analysis that can be used to determine the concentration of a known reactant. In part a, the concentration of naoh found in the first titration is 1/l and in the second titration the titration is 2/l. This will be a standard. Volume measurements play a key role in. In part b the. Volumetric Analysis Acid-Base Titration Lab Report.
From parkermcyrandolph.blogspot.com
Acid Base Titration Lab Report ParkermcyRandolph Volumetric Analysis Acid-Base Titration Lab Report In part b the concentration. A titration is a technique, in which a reagent, called a titrant, of known concentration is. For our experiment, the titrant will be a solution of potassium hydrogen phalate (khp) which is behaving as an acid. This will be a standard. In part a, the concentration of naoh found in the first titration is 1/l. Volumetric Analysis Acid-Base Titration Lab Report.
From www.chegg.com
Solved Volumetric Analysis AcidBase Titration Data Sheet Volumetric Analysis Acid-Base Titration Lab Report Those two concentrations are closed to one another but are not exact. For our experiment, the titrant will be a solution of potassium hydrogen phalate (khp) which is behaving as an acid. The goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via volumetric analysis, making use of the reaction of acetic acid with. Volumetric Analysis Acid-Base Titration Lab Report.
From www.docsity.com
Analysis of an Acid Base Titration Using the Gran Plot CHEM 321L Volumetric Analysis Acid-Base Titration Lab Report In part a, the concentration of naoh found in the first titration is 1/l and in the second titration the titration is 2/l. Volume measurements play a key role in. This will be a standard. For our experiment, the titrant will be a solution of potassium hydrogen phalate (khp) which is behaving as an acid. Those two concentrations are closed. Volumetric Analysis Acid-Base Titration Lab Report.
From collegedunia.com
Volumetric Analysis Titration, Types, Principle & Procedure Volumetric Analysis Acid-Base Titration Lab Report This will be a standard. A titration is a technique, in which a reagent, called a titrant, of known concentration is. Volume measurements play a key role in. The titrant is the substance in which we know the amount of moles. The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium hydroxide,. Volumetric Analysis Acid-Base Titration Lab Report.
From www.studocu.com
CHM1046 L Titration Part 1 Standardization of Na OH Volumetric Volumetric Analysis Acid-Base Titration Lab Report In part b the concentration. Titration is a common laboratory method of quantitative/chemical analysis that can be used to determine the concentration of a known reactant. In part a, the concentration of naoh found in the first titration is 1/l and in the second titration the titration is 2/l. Volume measurements play a key role in. This will be a. Volumetric Analysis Acid-Base Titration Lab Report.
From www.studypool.com
SOLUTION CHM 113 Experiment 8 Acid Base Titration Lab Report Studypool Volumetric Analysis Acid-Base Titration Lab Report The titrant is the substance in which we know the amount of moles. The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium hydroxide, a strong base and acetic acid, a weak acid with sodium hydroxide,. Titration is a common laboratory method of quantitative/chemical analysis that can be used to determine the. Volumetric Analysis Acid-Base Titration Lab Report.
From mavink.com
Acid Base Titration Lab Procedure Volumetric Analysis Acid-Base Titration Lab Report A titration is a technique, in which a reagent, called a titrant, of known concentration is. The goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via volumetric analysis, making use of the reaction of acetic acid with a strong base,. The titrant is the substance in which we know the amount of moles.. Volumetric Analysis Acid-Base Titration Lab Report.
From www.chegg.com
Solved REPORT SHEET Experiment 11 AcidBase Titration Volumetric Analysis Acid-Base Titration Lab Report Titration is a common laboratory method of quantitative/chemical analysis that can be used to determine the concentration of a known reactant. In part a, the concentration of naoh found in the first titration is 1/l and in the second titration the titration is 2/l. Volume measurements play a key role in. The goal of this experiment is to determine accurately. Volumetric Analysis Acid-Base Titration Lab Report.
From copywritersoffice.web.fc2.com
Order Your Own Writing Help Now volumetric analysis lab report 2017 Volumetric Analysis Acid-Base Titration Lab Report Volume measurements play a key role in. For our experiment, the titrant will be a solution of potassium hydrogen phalate (khp) which is behaving as an acid. In part b the concentration. The goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via volumetric analysis, making use of the reaction of acetic acid with. Volumetric Analysis Acid-Base Titration Lab Report.
From www.thinkswap.com
Lab report of volumetric analysis NT10402 Analytical Chemistry Volumetric Analysis Acid-Base Titration Lab Report Titration is a common laboratory method of quantitative/chemical analysis that can be used to determine the concentration of a known reactant. The goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via volumetric analysis, making use of the reaction of acetic acid with a strong base,. The titrant is the substance in which we. Volumetric Analysis Acid-Base Titration Lab Report.
From www.scribd.com
Lab Report Acid Base PDF Titration Chemistry Volumetric Analysis Acid-Base Titration Lab Report The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium hydroxide, a strong base and acetic acid, a weak acid with sodium hydroxide,. For our experiment, the titrant will be a solution of potassium hydrogen phalate (khp) which is behaving as an acid. Volume measurements play a key role in. This will. Volumetric Analysis Acid-Base Titration Lab Report.