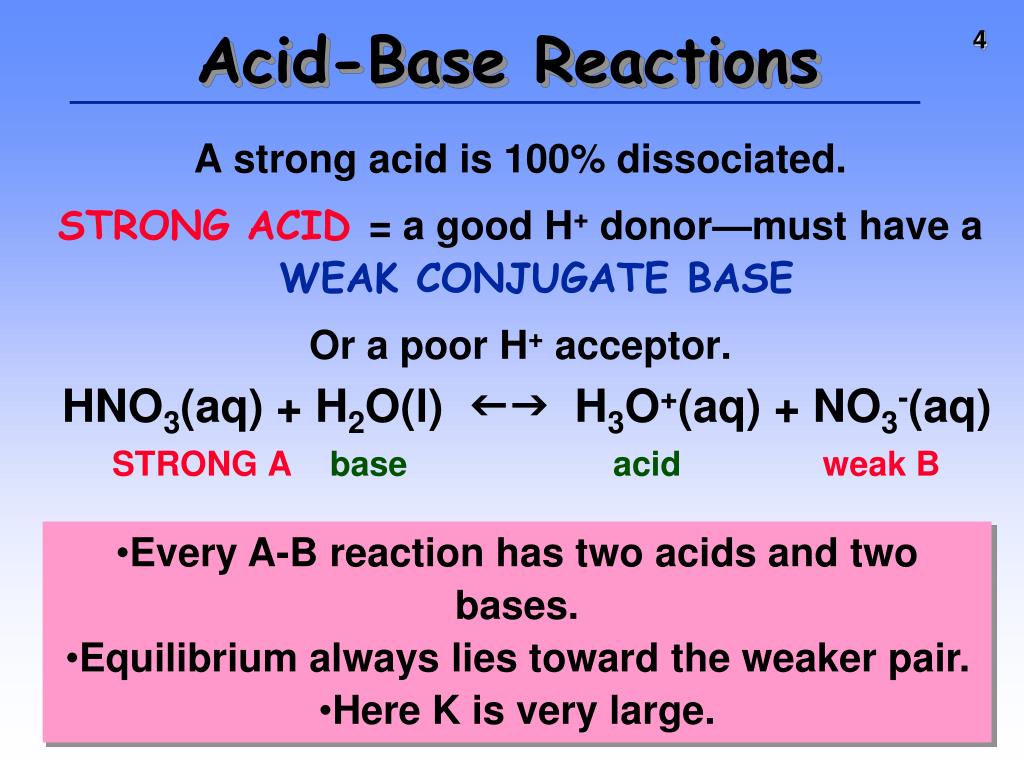
What Happens When You Add A Strong Base To A Strong Acid . See the graph of ph vs volume of titrant and the equivalence point at ph 7. Strong acid with strong base: The web page explains the concept of buffer capacity, buffer range, and buffer equation with examples and. A solution of acetic acid (\(\ce{ch3cooh}\). This leads to complete neutralization, forming a neutral salt and water. A strong base, unlike a weak base, dissociates (separates) completely into. Learn how to calculate the ph change during the titration of a strong acid with a strong base or vice versa. The acidity or basicity of an aqueous solution is described quantitatively using. Learn how buffers protect against ph changes when strong acid or base is added. The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. If you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other out and produce a salt and water. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \(\pageindex{1}\)). Mixing equal amounts of a. As we add strong base to a strong acid, the ph increases slowly until we near the equivalence point, where the ph increases. Hcl (acid) + naoh (base) → nacl (salt) + h₂o (water).
from www.slideserve.com
If you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other out and produce a salt and water. Learn how buffers protect against ph changes when strong acid or base is added. The acidity or basicity of an aqueous solution is described quantitatively using. Learn how to calculate the ph change during the titration of a strong acid with a strong base or vice versa. A solution of acetic acid (\(\ce{ch3cooh}\). A strong base, unlike a weak base, dissociates (separates) completely into. The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. See the graph of ph vs volume of titrant and the equivalence point at ph 7. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \(\pageindex{1}\)). As we add strong base to a strong acid, the ph increases slowly until we near the equivalence point, where the ph increases.
PPT AcidBase Reactions PowerPoint Presentation, free download ID3969900
What Happens When You Add A Strong Base To A Strong Acid Strong acid with strong base: The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. A solution of acetic acid (\(\ce{ch3cooh}\). Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \(\pageindex{1}\)). A strong base, unlike a weak base, dissociates (separates) completely into. Learn how buffers protect against ph changes when strong acid or base is added. This leads to complete neutralization, forming a neutral salt and water. See the graph of ph vs volume of titrant and the equivalence point at ph 7. The acidity or basicity of an aqueous solution is described quantitatively using. As we add strong base to a strong acid, the ph increases slowly until we near the equivalence point, where the ph increases. Hcl (acid) + naoh (base) → nacl (salt) + h₂o (water). If you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other out and produce a salt and water. Strong acid with strong base: Mixing equal amounts of a. Learn how to calculate the ph change during the titration of a strong acid with a strong base or vice versa. The web page explains the concept of buffer capacity, buffer range, and buffer equation with examples and.
From www.slideserve.com
PPT AcidBase Reactions PowerPoint Presentation, free download ID3969900 What Happens When You Add A Strong Base To A Strong Acid Mixing equal amounts of a. A solution of acetic acid (\(\ce{ch3cooh}\). As we add strong base to a strong acid, the ph increases slowly until we near the equivalence point, where the ph increases. The web page explains the concept of buffer capacity, buffer range, and buffer equation with examples and. Strong acid with strong base: Learn how buffers protect. What Happens When You Add A Strong Base To A Strong Acid.
From www.youtube.com
How to Remember the Strong Acids and Bases in 2 Minutes Easily YouTube What Happens When You Add A Strong Base To A Strong Acid If you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other out and produce a salt and water. The web page explains the concept of buffer capacity, buffer range, and buffer equation with examples and. The reaction of a strong acid with a strong base is a neutralization reaction, which produces. What Happens When You Add A Strong Base To A Strong Acid.
From www.slideserve.com
PPT Strong Acids & Bases PowerPoint Presentation, free download ID4664254 What Happens When You Add A Strong Base To A Strong Acid Learn how to calculate the ph change during the titration of a strong acid with a strong base or vice versa. Strong acid with strong base: Hcl (acid) + naoh (base) → nacl (salt) + h₂o (water). The web page explains the concept of buffer capacity, buffer range, and buffer equation with examples and. Buffer solutions resist a change in. What Happens When You Add A Strong Base To A Strong Acid.
From www.chemicals.co.uk
A Level Chemistry Revision Physical Chemistry Acids And Bases What Happens When You Add A Strong Base To A Strong Acid If you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other out and produce a salt and water. The web page explains the concept of buffer capacity, buffer range, and buffer equation with examples and. Strong acid with strong base: A solution of acetic acid (\(\ce{ch3cooh}\). The reaction of a strong. What Happens When You Add A Strong Base To A Strong Acid.
From stock.adobe.com
Strong acid and strong base reaction. Strengths of acids and bases. Scientific vector What Happens When You Add A Strong Base To A Strong Acid If you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other out and produce a salt and water. The acidity or basicity of an aqueous solution is described quantitatively using. Hcl (acid) + naoh (base) → nacl (salt) + h₂o (water). Learn how buffers protect against ph changes when strong acid. What Happens When You Add A Strong Base To A Strong Acid.
From www.slideserve.com
PPT Acids & Bases PowerPoint Presentation ID6155898 What Happens When You Add A Strong Base To A Strong Acid Hcl (acid) + naoh (base) → nacl (salt) + h₂o (water). A solution of acetic acid (\(\ce{ch3cooh}\). See the graph of ph vs volume of titrant and the equivalence point at ph 7. The acidity or basicity of an aqueous solution is described quantitatively using. If you mix equal amounts of a strong acid and a strong base, the two. What Happens When You Add A Strong Base To A Strong Acid.
From www.teachoo.com
List of Strong Acids (More than 8 examples, with images) Teachoo What Happens When You Add A Strong Base To A Strong Acid The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. This leads to complete neutralization, forming a neutral salt and water. A strong base, unlike a weak base, dissociates (separates) completely into. Learn how buffers protect against ph changes when strong acid or base is added. If you mix equal. What Happens When You Add A Strong Base To A Strong Acid.
From www.slideserve.com
PPT Common Strong acids PowerPoint Presentation, free download ID4763569 What Happens When You Add A Strong Base To A Strong Acid As we add strong base to a strong acid, the ph increases slowly until we near the equivalence point, where the ph increases. Hcl (acid) + naoh (base) → nacl (salt) + h₂o (water). Mixing equal amounts of a. Strong acid with strong base: See the graph of ph vs volume of titrant and the equivalence point at ph 7.. What Happens When You Add A Strong Base To A Strong Acid.
From www.youtube.com
Strong Acids and Strong Bases YouTube What Happens When You Add A Strong Base To A Strong Acid If you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other out and produce a salt and water. A solution of acetic acid (\(\ce{ch3cooh}\). The acidity or basicity of an aqueous solution is described quantitatively using. Mixing equal amounts of a. A strong base, unlike a weak base, dissociates (separates) completely. What Happens When You Add A Strong Base To A Strong Acid.
From www.flinnsci.ca
AcidBase Strength Charts for Chemistry What Happens When You Add A Strong Base To A Strong Acid The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \(\pageindex{1}\)). Hcl (acid) + naoh (base) → nacl (salt) + h₂o (water). If you mix equal amounts of a. What Happens When You Add A Strong Base To A Strong Acid.
From www.youtube.com
ACID AND BASE COMPLETE EXPLAIN Strong acid and weak acid Strong base and weak base What Happens When You Add A Strong Base To A Strong Acid If you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other out and produce a salt and water. This leads to complete neutralization, forming a neutral salt and water. Hcl (acid) + naoh (base) → nacl (salt) + h₂o (water). As we add strong base to a strong acid, the ph. What Happens When You Add A Strong Base To A Strong Acid.
From www.slideserve.com
PPT MODERN CHEMISTRY CHAPTER 14 ACIDS AND BASES PowerPoint Presentation ID269675 What Happens When You Add A Strong Base To A Strong Acid Learn how to calculate the ph change during the titration of a strong acid with a strong base or vice versa. Learn how buffers protect against ph changes when strong acid or base is added. The web page explains the concept of buffer capacity, buffer range, and buffer equation with examples and. A solution of acetic acid (\(\ce{ch3cooh}\). Mixing equal. What Happens When You Add A Strong Base To A Strong Acid.
From www.slideserve.com
PPT Acid Base Reactions PowerPoint Presentation, free download ID3780900 What Happens When You Add A Strong Base To A Strong Acid Learn how buffers protect against ph changes when strong acid or base is added. If you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other out and produce a salt and water. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are. What Happens When You Add A Strong Base To A Strong Acid.
From ar.inspiredpencil.com
A Weak Base Strong Acid Weak Base With A Combines To Form What Happens When You Add A Strong Base To A Strong Acid Learn how to calculate the ph change during the titration of a strong acid with a strong base or vice versa. As we add strong base to a strong acid, the ph increases slowly until we near the equivalence point, where the ph increases. A solution of acetic acid (\(\ce{ch3cooh}\). If you mix equal amounts of a strong acid and. What Happens When You Add A Strong Base To A Strong Acid.
From www.slideserve.com
PPT Ionization Constants of Acids and Bases Strengths of Acids and Bases PowerPoint What Happens When You Add A Strong Base To A Strong Acid As we add strong base to a strong acid, the ph increases slowly until we near the equivalence point, where the ph increases. Hcl (acid) + naoh (base) → nacl (salt) + h₂o (water). The acidity or basicity of an aqueous solution is described quantitatively using. If you mix equal amounts of a strong acid and a strong base, the. What Happens When You Add A Strong Base To A Strong Acid.
From www.youtube.com
Weak basestrong acid reactions Acids and bases AP Chemistry Khan Academy YouTube What Happens When You Add A Strong Base To A Strong Acid Learn how to calculate the ph change during the titration of a strong acid with a strong base or vice versa. Strong acid with strong base: A strong base, unlike a weak base, dissociates (separates) completely into. Learn how buffers protect against ph changes when strong acid or base is added. Mixing equal amounts of a. The reaction of a. What Happens When You Add A Strong Base To A Strong Acid.
From www.youtube.com
Effect of adding Water on the pH of the Strong Acid YouTube What Happens When You Add A Strong Base To A Strong Acid If you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other out and produce a salt and water. The acidity or basicity of an aqueous solution is described quantitatively using. Learn how to calculate the ph change during the titration of a strong acid with a strong base or vice versa.. What Happens When You Add A Strong Base To A Strong Acid.
From www.youtube.com
What happens to and Acid or Base in Water Solution?Acid Bases and Salts Class 10 PART 8 What Happens When You Add A Strong Base To A Strong Acid A strong base, unlike a weak base, dissociates (separates) completely into. Mixing equal amounts of a. The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. If you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other out and produce a. What Happens When You Add A Strong Base To A Strong Acid.
From www.slideserve.com
PPT MODERN CHEMISTRY CHAPTER 14 ACIDS AND BASES PowerPoint Presentation ID269675 What Happens When You Add A Strong Base To A Strong Acid See the graph of ph vs volume of titrant and the equivalence point at ph 7. Mixing equal amounts of a. The web page explains the concept of buffer capacity, buffer range, and buffer equation with examples and. The acidity or basicity of an aqueous solution is described quantitatively using. Buffer solutions resist a change in ph when small amounts. What Happens When You Add A Strong Base To A Strong Acid.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps What Happens When You Add A Strong Base To A Strong Acid Mixing equal amounts of a. If you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other out and produce a salt and water. The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. Buffer solutions resist a change in ph when. What Happens When You Add A Strong Base To A Strong Acid.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts What Happens When You Add A Strong Base To A Strong Acid Learn how to calculate the ph change during the titration of a strong acid with a strong base or vice versa. Mixing equal amounts of a. This leads to complete neutralization, forming a neutral salt and water. A solution of acetic acid (\(\ce{ch3cooh}\). Hcl (acid) + naoh (base) → nacl (salt) + h₂o (water). If you mix equal amounts of. What Happens When You Add A Strong Base To A Strong Acid.
From www.youtube.com
Mixing Strong Concentrated Acid and Base YouTube What Happens When You Add A Strong Base To A Strong Acid A solution of acetic acid (\(\ce{ch3cooh}\). Hcl (acid) + naoh (base) → nacl (salt) + h₂o (water). As we add strong base to a strong acid, the ph increases slowly until we near the equivalence point, where the ph increases. The web page explains the concept of buffer capacity, buffer range, and buffer equation with examples and. Strong acid with. What Happens When You Add A Strong Base To A Strong Acid.
From www.thoughtco.com
List of the Strong Bases (Arrhenius Bases) What Happens When You Add A Strong Base To A Strong Acid A solution of acetic acid (\(\ce{ch3cooh}\). A strong base, unlike a weak base, dissociates (separates) completely into. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \(\pageindex{1}\)). Learn how to calculate the ph change during the titration of a strong acid with a strong base or vice versa.. What Happens When You Add A Strong Base To A Strong Acid.
From www.youtube.com
AP chemistry Adding Strong Acid to Buffer problem YouTube What Happens When You Add A Strong Base To A Strong Acid See the graph of ph vs volume of titrant and the equivalence point at ph 7. Learn how to calculate the ph change during the titration of a strong acid with a strong base or vice versa. A solution of acetic acid (\(\ce{ch3cooh}\). As we add strong base to a strong acid, the ph increases slowly until we near the. What Happens When You Add A Strong Base To A Strong Acid.
From www.slideserve.com
PPT AcidBase Reactions PowerPoint Presentation, free download ID3969900 What Happens When You Add A Strong Base To A Strong Acid Learn how buffers protect against ph changes when strong acid or base is added. This leads to complete neutralization, forming a neutral salt and water. See the graph of ph vs volume of titrant and the equivalence point at ph 7. Learn how to calculate the ph change during the titration of a strong acid with a strong base or. What Happens When You Add A Strong Base To A Strong Acid.
From www.pinterest.com
Strong and Weak Acids & Bases Chemistry classroom, Ap chemistry, Teaching science What Happens When You Add A Strong Base To A Strong Acid As we add strong base to a strong acid, the ph increases slowly until we near the equivalence point, where the ph increases. Strong acid with strong base: Learn how buffers protect against ph changes when strong acid or base is added. Mixing equal amounts of a. The acidity or basicity of an aqueous solution is described quantitatively using. A. What Happens When You Add A Strong Base To A Strong Acid.
From www.slideserve.com
PPT Strong Acids and Bases PowerPoint Presentation, free download ID2617724 What Happens When You Add A Strong Base To A Strong Acid Learn how buffers protect against ph changes when strong acid or base is added. Mixing equal amounts of a. The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. This leads to complete neutralization, forming a neutral salt and water. As we add strong base to a strong acid, the. What Happens When You Add A Strong Base To A Strong Acid.
From www.teachoo.com
List of Strong Bases 7+ Examples Chemistry Teachoo What Happens When You Add A Strong Base To A Strong Acid The web page explains the concept of buffer capacity, buffer range, and buffer equation with examples and. Hcl (acid) + naoh (base) → nacl (salt) + h₂o (water). Mixing equal amounts of a. See the graph of ph vs volume of titrant and the equivalence point at ph 7. Learn how buffers protect against ph changes when strong acid or. What Happens When You Add A Strong Base To A Strong Acid.
From www.masterorganicchemistry.com
How To Use a pKa Table What Happens When You Add A Strong Base To A Strong Acid This leads to complete neutralization, forming a neutral salt and water. Learn how buffers protect against ph changes when strong acid or base is added. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \(\pageindex{1}\)). Mixing equal amounts of a. A strong base, unlike a weak base, dissociates. What Happens When You Add A Strong Base To A Strong Acid.
From www.slideserve.com
PPT Topic 18 Acids and bases PowerPoint Presentation, free download ID1852384 What Happens When You Add A Strong Base To A Strong Acid As we add strong base to a strong acid, the ph increases slowly until we near the equivalence point, where the ph increases. The acidity or basicity of an aqueous solution is described quantitatively using. Learn how to calculate the ph change during the titration of a strong acid with a strong base or vice versa. Hcl (acid) + naoh. What Happens When You Add A Strong Base To A Strong Acid.
From stock.adobe.com
Strong acid and strong base reaction. Strengths of acids and bases. Scientific vector What Happens When You Add A Strong Base To A Strong Acid Hcl (acid) + naoh (base) → nacl (salt) + h₂o (water). Strong acid with strong base: Learn how buffers protect against ph changes when strong acid or base is added. A solution of acetic acid (\(\ce{ch3cooh}\). Learn how to calculate the ph change during the titration of a strong acid with a strong base or vice versa. Buffer solutions resist. What Happens When You Add A Strong Base To A Strong Acid.
From www.youtube.com
How To Memorize The Strong Acids and Strong Bases YouTube What Happens When You Add A Strong Base To A Strong Acid The acidity or basicity of an aqueous solution is described quantitatively using. Mixing equal amounts of a. Hcl (acid) + naoh (base) → nacl (salt) + h₂o (water). A strong base, unlike a weak base, dissociates (separates) completely into. This leads to complete neutralization, forming a neutral salt and water. Learn how buffers protect against ph changes when strong acid. What Happens When You Add A Strong Base To A Strong Acid.
From www.slideshare.net
1 22 What Are Strong Acids And Bases What Happens When You Add A Strong Base To A Strong Acid If you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other out and produce a salt and water. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \(\pageindex{1}\)). This leads to complete neutralization, forming a neutral salt and water.. What Happens When You Add A Strong Base To A Strong Acid.
From www.khanacademy.org
Acidbase reactions (practice) Khan Academy What Happens When You Add A Strong Base To A Strong Acid Hcl (acid) + naoh (base) → nacl (salt) + h₂o (water). Learn how buffers protect against ph changes when strong acid or base is added. This leads to complete neutralization, forming a neutral salt and water. A solution of acetic acid (\(\ce{ch3cooh}\). The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus. What Happens When You Add A Strong Base To A Strong Acid.
From www.youtube.com
Strong acidstrong base reactions Acids and bases AP Chemistry Khan Academy YouTube What Happens When You Add A Strong Base To A Strong Acid A strong base, unlike a weak base, dissociates (separates) completely into. This leads to complete neutralization, forming a neutral salt and water. If you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other out and produce a salt and water. Strong acid with strong base: See the graph of ph vs. What Happens When You Add A Strong Base To A Strong Acid.