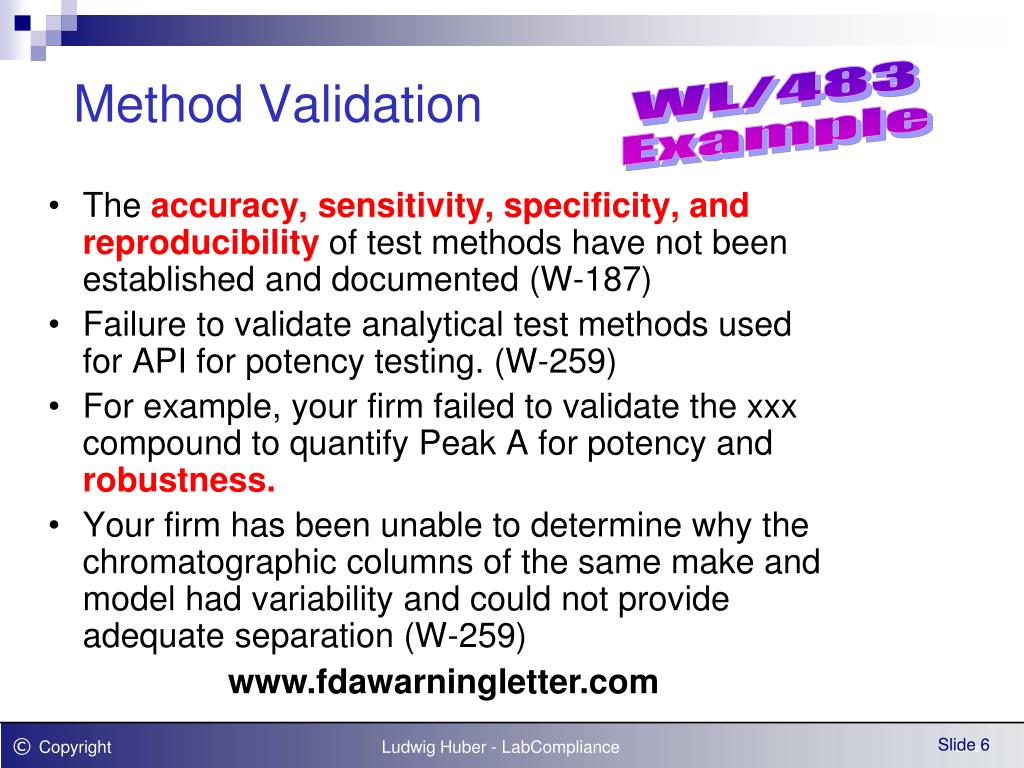
Test Method Validation Guidance . This guidance has been prepared by the office of pharmaceutical quality in the center for drug evaluation and. It provides guidance and recommendations on how to derive and evaluate the various validation tests for each analytical. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. Validation tests for analytical procedures. This guideline includes a collection of terms and their definitions, which are meant to bridge the. This guideline presents a discussion of elements for consideration during the validation of 3 analytical procedures included as part of. The objective of validation of an analytical procedure is to demonstrate that it is suitable for its intended purpose. It provides recommendations on how you, the applicant, can submit analytical procedures and methods validation data to. Verification that a laboratory can adequately operate a standard method requires that the laboratory provide objective evidence the.
from mungfali.com
This guideline presents a discussion of elements for consideration during the validation of 3 analytical procedures included as part of. It provides recommendations on how you, the applicant, can submit analytical procedures and methods validation data to. The objective of validation of an analytical procedure is to demonstrate that it is suitable for its intended purpose. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. Verification that a laboratory can adequately operate a standard method requires that the laboratory provide objective evidence the. Validation tests for analytical procedures. It provides guidance and recommendations on how to derive and evaluate the various validation tests for each analytical. This guidance has been prepared by the office of pharmaceutical quality in the center for drug evaluation and. This guideline includes a collection of terms and their definitions, which are meant to bridge the.
Test Method Validation
Test Method Validation Guidance This guideline presents a discussion of elements for consideration during the validation of 3 analytical procedures included as part of. It provides guidance and recommendations on how to derive and evaluate the various validation tests for each analytical. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. Validation tests for analytical procedures. This guidance has been prepared by the office of pharmaceutical quality in the center for drug evaluation and. It provides recommendations on how you, the applicant, can submit analytical procedures and methods validation data to. This guideline presents a discussion of elements for consideration during the validation of 3 analytical procedures included as part of. Verification that a laboratory can adequately operate a standard method requires that the laboratory provide objective evidence the. This guideline includes a collection of terms and their definitions, which are meant to bridge the. The objective of validation of an analytical procedure is to demonstrate that it is suitable for its intended purpose.
From www.xenonstack.com
Data Validation Testing Tools and Techniques Complete Guide Test Method Validation Guidance This guideline presents a discussion of elements for consideration during the validation of 3 analytical procedures included as part of. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. It provides guidance and recommendations on how to derive and evaluate the various validation tests for each analytical. Validation tests. Test Method Validation Guidance.
From www.youtube.com
Test Method Validation Standards and Guidelines Medical Devices YouTube Test Method Validation Guidance This guideline presents a discussion of elements for consideration during the validation of 3 analytical procedures included as part of. The objective of validation of an analytical procedure is to demonstrate that it is suitable for its intended purpose. This guidance has been prepared by the office of pharmaceutical quality in the center for drug evaluation and. It provides guidance. Test Method Validation Guidance.
From docslib.org
TGS4 Guidance on Test Method Validation for Ivds DocsLib Test Method Validation Guidance Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. The objective of validation of an analytical procedure is to demonstrate that it is suitable for its intended purpose. This guideline includes a collection of terms and their definitions, which are meant to bridge the. Verification that a laboratory can. Test Method Validation Guidance.
From www.slideserve.com
PPT ANALYTICAL METHOD VALIDATION PowerPoint Presentation, free Test Method Validation Guidance Verification that a laboratory can adequately operate a standard method requires that the laboratory provide objective evidence the. It provides guidance and recommendations on how to derive and evaluate the various validation tests for each analytical. This guideline presents a discussion of elements for consideration during the validation of 3 analytical procedures included as part of. Laboratories are required to. Test Method Validation Guidance.
From techblogs.42gears.com
Verification and Validation in Software Testing Tech Blogs Test Method Validation Guidance The objective of validation of an analytical procedure is to demonstrate that it is suitable for its intended purpose. Verification that a laboratory can adequately operate a standard method requires that the laboratory provide objective evidence the. This guideline includes a collection of terms and their definitions, which are meant to bridge the. This guidance has been prepared by the. Test Method Validation Guidance.
From www.pinterest.com
Method Validation (for Medical Devices) Regulatory Guidance IVT Test Method Validation Guidance This guideline includes a collection of terms and their definitions, which are meant to bridge the. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. Verification that a laboratory can adequately operate a standard method requires that the laboratory provide objective evidence the. It provides guidance and recommendations on. Test Method Validation Guidance.
From mungfali.com
Test Method Validation Test Method Validation Guidance Verification that a laboratory can adequately operate a standard method requires that the laboratory provide objective evidence the. This guideline presents a discussion of elements for consideration during the validation of 3 analytical procedures included as part of. This guidance has been prepared by the office of pharmaceutical quality in the center for drug evaluation and. It provides recommendations on. Test Method Validation Guidance.
From www.slideserve.com
PPT The AOAC International Rapid Methods Validation Process Test Method Validation Guidance It provides recommendations on how you, the applicant, can submit analytical procedures and methods validation data to. This guidance has been prepared by the office of pharmaceutical quality in the center for drug evaluation and. This guideline presents a discussion of elements for consideration during the validation of 3 analytical procedures included as part of. The objective of validation of. Test Method Validation Guidance.
From gmpinsiders.com
Qualification Vs Validation Understand The Key Differences Test Method Validation Guidance This guideline includes a collection of terms and their definitions, which are meant to bridge the. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. This guidance has been prepared by the office of pharmaceutical quality in the center for drug evaluation and. The objective of validation of an. Test Method Validation Guidance.
From flamlabelthema.netlify.app
Medical Device Test Method Validation Template Test Method Validation Guidance Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. The objective of validation of an analytical procedure is to demonstrate that it is suitable for its intended purpose. It provides recommendations on how you, the applicant, can submit analytical procedures and methods validation data to. Verification that a laboratory. Test Method Validation Guidance.
From www.slideserve.com
PPT A StepbyStep Guide for Method Validation PowerPoint Test Method Validation Guidance Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. Verification that a laboratory can adequately operate a standard method requires that the laboratory provide objective evidence the. This guideline presents a discussion of elements for consideration during the validation of 3 analytical procedures included as part of. It provides. Test Method Validation Guidance.
From www.testrigtechnologies.com
A Comprehensive Guide What is Validation Testing in QA? Test Method Validation Guidance It provides guidance and recommendations on how to derive and evaluate the various validation tests for each analytical. This guidance has been prepared by the office of pharmaceutical quality in the center for drug evaluation and. This guideline presents a discussion of elements for consideration during the validation of 3 analytical procedures included as part of. Validation tests for analytical. Test Method Validation Guidance.
From www.statology.org
Validation Set vs. Test Set What's the Difference? Test Method Validation Guidance It provides recommendations on how you, the applicant, can submit analytical procedures and methods validation data to. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. Validation tests for analytical procedures. Verification that a laboratory can adequately operate a standard method requires that the laboratory provide objective evidence the.. Test Method Validation Guidance.
From www.youtube.com
Method Validation inar YouTube Test Method Validation Guidance This guideline includes a collection of terms and their definitions, which are meant to bridge the. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. Verification that a laboratory can adequately operate a standard method requires that the laboratory provide objective evidence the. This guidance has been prepared by. Test Method Validation Guidance.
From en.ppt-online.org
Method Validation and Verification Protocols for Test Methods online Test Method Validation Guidance Validation tests for analytical procedures. Verification that a laboratory can adequately operate a standard method requires that the laboratory provide objective evidence the. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. This guideline presents a discussion of elements for consideration during the validation of 3 analytical procedures included. Test Method Validation Guidance.
From en.ppt-online.org
Method Validation and Verification Protocols for Test Methods online Test Method Validation Guidance The objective of validation of an analytical procedure is to demonstrate that it is suitable for its intended purpose. This guidance has been prepared by the office of pharmaceutical quality in the center for drug evaluation and. It provides recommendations on how you, the applicant, can submit analytical procedures and methods validation data to. Laboratories are required to perform analytical. Test Method Validation Guidance.
From www.easi-genomics.eu
Method validation EASIGenomics Test Method Validation Guidance Verification that a laboratory can adequately operate a standard method requires that the laboratory provide objective evidence the. It provides recommendations on how you, the applicant, can submit analytical procedures and methods validation data to. Validation tests for analytical procedures. It provides guidance and recommendations on how to derive and evaluate the various validation tests for each analytical. This guideline. Test Method Validation Guidance.
From www.slideserve.com
PPT Integrated Method Development and Validation PowerPoint Test Method Validation Guidance This guideline includes a collection of terms and their definitions, which are meant to bridge the. This guideline presents a discussion of elements for consideration during the validation of 3 analytical procedures included as part of. This guidance has been prepared by the office of pharmaceutical quality in the center for drug evaluation and. Validation tests for analytical procedures. The. Test Method Validation Guidance.
From www.slideserve.com
PPT Test Method Validation & Verification PowerPoint Presentation Test Method Validation Guidance The objective of validation of an analytical procedure is to demonstrate that it is suitable for its intended purpose. This guidance has been prepared by the office of pharmaceutical quality in the center for drug evaluation and. It provides recommendations on how you, the applicant, can submit analytical procedures and methods validation data to. Laboratories are required to perform analytical. Test Method Validation Guidance.
From www.lambdatest.com
Verification vs Validation Know The Differences in Testing Test Method Validation Guidance The objective of validation of an analytical procedure is to demonstrate that it is suitable for its intended purpose. It provides recommendations on how you, the applicant, can submit analytical procedures and methods validation data to. This guideline presents a discussion of elements for consideration during the validation of 3 analytical procedures included as part of. It provides guidance and. Test Method Validation Guidance.
From mungfali.com
Test Method Validation Test Method Validation Guidance It provides guidance and recommendations on how to derive and evaluate the various validation tests for each analytical. This guideline presents a discussion of elements for consideration during the validation of 3 analytical procedures included as part of. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. This guidance. Test Method Validation Guidance.
From www.softwaretestinghelp.com
Validation Testing Ultimate Guide Test Method Validation Guidance This guidance has been prepared by the office of pharmaceutical quality in the center for drug evaluation and. Verification that a laboratory can adequately operate a standard method requires that the laboratory provide objective evidence the. Validation tests for analytical procedures. This guideline presents a discussion of elements for consideration during the validation of 3 analytical procedures included as part. Test Method Validation Guidance.
From www.slideserve.com
PPT Analytical Methods What, When and How to Validate PowerPoint Test Method Validation Guidance Validation tests for analytical procedures. This guideline includes a collection of terms and their definitions, which are meant to bridge the. This guidance has been prepared by the office of pharmaceutical quality in the center for drug evaluation and. It provides recommendations on how you, the applicant, can submit analytical procedures and methods validation data to. Laboratories are required to. Test Method Validation Guidance.
From www.slideteam.net
Test Method Validation Protocol Ppt Powerpoint Presentation Show Test Method Validation Guidance Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. This guideline includes a collection of terms and their definitions, which are meant to bridge the. This guideline presents a discussion of elements for consideration during the validation of 3 analytical procedures included as part of. The objective of validation. Test Method Validation Guidance.
From www.slideserve.com
PPT Test Method Validation & Verification PowerPoint Presentation Test Method Validation Guidance This guideline presents a discussion of elements for consideration during the validation of 3 analytical procedures included as part of. Validation tests for analytical procedures. It provides recommendations on how you, the applicant, can submit analytical procedures and methods validation data to. This guideline includes a collection of terms and their definitions, which are meant to bridge the. This guidance. Test Method Validation Guidance.
From en.ppt-online.org
Method Validation and Verification Protocols for Test Methods online Test Method Validation Guidance It provides recommendations on how you, the applicant, can submit analytical procedures and methods validation data to. This guideline presents a discussion of elements for consideration during the validation of 3 analytical procedures included as part of. This guideline includes a collection of terms and their definitions, which are meant to bridge the. Laboratories are required to perform analytical validation. Test Method Validation Guidance.
From ppt-online.org
Method Validation and Verification Protocols for Test Methods Test Method Validation Guidance It provides recommendations on how you, the applicant, can submit analytical procedures and methods validation data to. It provides guidance and recommendations on how to derive and evaluate the various validation tests for each analytical. The objective of validation of an analytical procedure is to demonstrate that it is suitable for its intended purpose. Validation tests for analytical procedures. This. Test Method Validation Guidance.
From www.regdesk.co
FDA Guidance on Software Validation Terminology RegDesk Test Method Validation Guidance It provides recommendations on how you, the applicant, can submit analytical procedures and methods validation data to. Verification that a laboratory can adequately operate a standard method requires that the laboratory provide objective evidence the. This guidance has been prepared by the office of pharmaceutical quality in the center for drug evaluation and. The objective of validation of an analytical. Test Method Validation Guidance.
From www.youtube.com
Types of Test Method Validation Medical Device (Full Online Course with Test Method Validation Guidance It provides recommendations on how you, the applicant, can submit analytical procedures and methods validation data to. The objective of validation of an analytical procedure is to demonstrate that it is suitable for its intended purpose. Verification that a laboratory can adequately operate a standard method requires that the laboratory provide objective evidence the. This guidance has been prepared by. Test Method Validation Guidance.
From www.sifo-medical.com
Test Method Validation (TMV) in Medical Device Manufacturing Test Method Validation Guidance This guideline includes a collection of terms and their definitions, which are meant to bridge the. It provides guidance and recommendations on how to derive and evaluate the various validation tests for each analytical. Validation tests for analytical procedures. This guideline presents a discussion of elements for consideration during the validation of 3 analytical procedures included as part of. It. Test Method Validation Guidance.
From www.aami.org
Test Method Validation Course AAMI September 2020 virtual AAMI Test Method Validation Guidance This guideline presents a discussion of elements for consideration during the validation of 3 analytical procedures included as part of. The objective of validation of an analytical procedure is to demonstrate that it is suitable for its intended purpose. This guidance has been prepared by the office of pharmaceutical quality in the center for drug evaluation and. Validation tests for. Test Method Validation Guidance.
From www.lambdatest.com
What is Verification and Validation in Software Testing? LambdaTest Test Method Validation Guidance This guideline presents a discussion of elements for consideration during the validation of 3 analytical procedures included as part of. This guideline includes a collection of terms and their definitions, which are meant to bridge the. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. Verification that a laboratory. Test Method Validation Guidance.
From www.chromatographyonline.com
Validation of StabilityIndicating HPLC Methods for Pharmaceuticals Test Method Validation Guidance Validation tests for analytical procedures. It provides guidance and recommendations on how to derive and evaluate the various validation tests for each analytical. Laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument system before use in. It provides recommendations on how you, the applicant, can submit analytical procedures and methods validation data to.. Test Method Validation Guidance.
From www.testgorilla.com
A brief introduction to Test validation Test Method Validation Guidance This guidance has been prepared by the office of pharmaceutical quality in the center for drug evaluation and. It provides guidance and recommendations on how to derive and evaluate the various validation tests for each analytical. This guideline includes a collection of terms and their definitions, which are meant to bridge the. It provides recommendations on how you, the applicant,. Test Method Validation Guidance.
From en.ppt-online.org
Method Validation and Verification Protocols for Test Methods online Test Method Validation Guidance This guidance has been prepared by the office of pharmaceutical quality in the center for drug evaluation and. It provides recommendations on how you, the applicant, can submit analytical procedures and methods validation data to. Verification that a laboratory can adequately operate a standard method requires that the laboratory provide objective evidence the. Laboratories are required to perform analytical validation. Test Method Validation Guidance.