Graph Of Calorimetry . A doubled styrofoam cup fitted with a cover in which a hole. Find the heat capacity (cp) of a calorimeter and contents (calibration). A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calculating the enthalpy change of reaction, hrfrom experimental data. A calorimeter is a device used to determine heat flow during a chemical or physical change. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calorimetry is the measurement enthalpy changes in chemical reactions. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. Measurement of an enthalpy change. There are two types of calorimetry experiments you need to know:. For example, when an exothermic. This experiment has three primary objectives: Determine the hrxn, the enthalpy of reaction, in kj/mol for several different reactions,. Apply the first law of thermodynamics to calorimetry.
from wisc.pb.unizin.org
A doubled styrofoam cup fitted with a cover in which a hole. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. A calorimeter is a device used to determine heat flow during a chemical or physical change. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. There are two types of calorimetry experiments you need to know:. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. Calorimetry is the measurement enthalpy changes in chemical reactions. This experiment has three primary objectives: Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Determine the hrxn, the enthalpy of reaction, in kj/mol for several different reactions,.
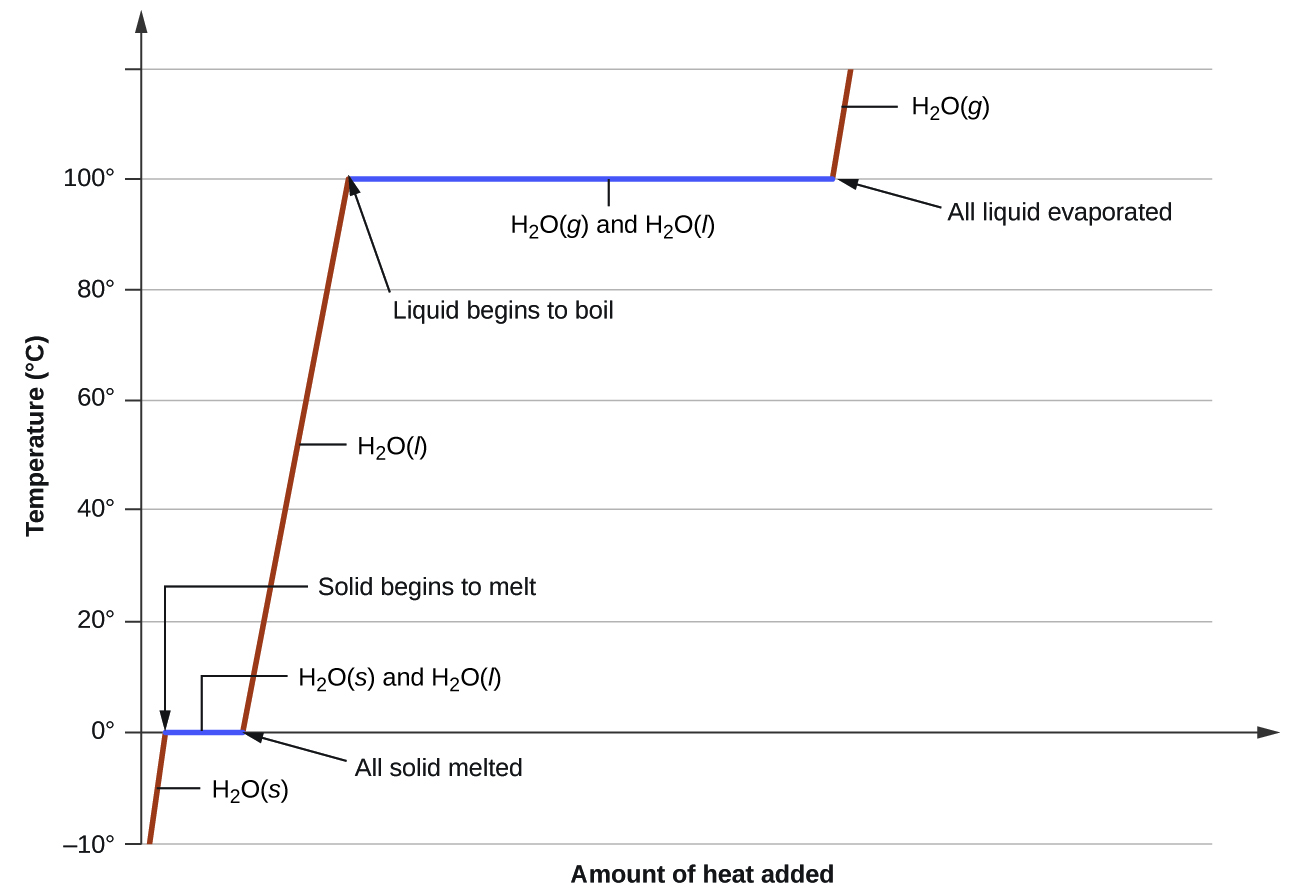
Calorimetry continued Phase Changes and Heating Curves (M6Q6) UW
Graph Of Calorimetry A doubled styrofoam cup fitted with a cover in which a hole. For example, when an exothermic. Apply the first law of thermodynamics to calorimetry. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. Calorimetry is the measurement enthalpy changes in chemical reactions. Determine the hrxn, the enthalpy of reaction, in kj/mol for several different reactions,. A doubled styrofoam cup fitted with a cover in which a hole. Calculating the enthalpy change of reaction, hrfrom experimental data. There are two types of calorimetry experiments you need to know:. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. This experiment has three primary objectives: A calorimeter is a device used to determine heat flow during a chemical or physical change. Measurement of an enthalpy change. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Find the heat capacity (cp) of a calorimeter and contents (calibration).
From www.youtube.com
How do you measure DeltaH? calorimetry YouTube Graph Of Calorimetry A calorimeter is a device used to determine heat flow during a chemical or physical change. Calorimetry is the measurement enthalpy changes in chemical reactions. This experiment has three primary objectives: For example, when an exothermic. There are two types of calorimetry experiments you need to know:. Calculating the enthalpy change of reaction, hrfrom experimental data. Use our revision notes. Graph Of Calorimetry.
From covalentmetrology.com
Differential Scanning Calorimetry (DSC) Covalent Metrology Graph Of Calorimetry There are two types of calorimetry experiments you need to know:. A calorimeter is a device used to determine heat flow during a chemical or physical change. This experiment has three primary objectives: For example, when an exothermic. Measurement of an enthalpy change. Calorimetry is the measurement enthalpy changes in chemical reactions. Apply the first law of thermodynamics to calorimetry.. Graph Of Calorimetry.
From klacxfopf.blob.core.windows.net
Graph For Calorimetry at Loraine Koo blog Graph Of Calorimetry A doubled styrofoam cup fitted with a cover in which a hole. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. There are two types of calorimetry experiments you need to know:. Find the heat capacity (cp) of a calorimeter and contents (calibration). Calculate heat, temperature change, and specific heat after thermal equilibrium. Graph Of Calorimetry.
From www.researchgate.net
Differential scanning calorimetry (DSC) curves of STPU(PCL)0.9/1.1 Graph Of Calorimetry Measurement of an enthalpy change. Determine the hrxn, the enthalpy of reaction, in kj/mol for several different reactions,. Calculating the enthalpy change of reaction, hrfrom experimental data. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. A calorimeter is a device used to determine heat flow during a chemical or physical. Graph Of Calorimetry.
From www.researchgate.net
Thermogravimetrydifferential scanning calorimetry (TGDSC) curves of Graph Of Calorimetry Calorimetry is the measurement enthalpy changes in chemical reactions. This experiment has three primary objectives: A calorimeter is a device used to determine heat flow during a chemical or physical change. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. Measurement of an enthalpy change. For example,. Graph Of Calorimetry.
From www.researchgate.net
Differential scanning calorimetry (DSC) curve. Download Scientific Graph Of Calorimetry Determine the hrxn, the enthalpy of reaction, in kj/mol for several different reactions,. Calorimetry is the measurement enthalpy changes in chemical reactions. Measurement of an enthalpy change. Find the heat capacity (cp) of a calorimeter and contents (calibration). There are two types of calorimetry experiments you need to know:. Compare heat flow from hot to cold objects in an ideal. Graph Of Calorimetry.
From chem.libretexts.org
Differential Scanning Calorimetry Chemistry LibreTexts Graph Of Calorimetry Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Determine the hrxn, the enthalpy of reaction, in kj/mol for several different reactions,. Calculating the enthalpy change of reaction, hrfrom experimental data. A doubled styrofoam cup fitted with a cover in which a hole. For example, when an exothermic. Use our revision notes on. Graph Of Calorimetry.
From www.researchgate.net
( a ) Representative differential scanning calorimetry graph of the Graph Of Calorimetry There are two types of calorimetry experiments you need to know:. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used to determine heat flow during a chemical or physical change. Determine the hrxn, the enthalpy of reaction,. Graph Of Calorimetry.
From www.savemyexams.com
Calorimetry Experiments SL IB Chemistry Revision Notes 2025 Graph Of Calorimetry Determine the hrxn, the enthalpy of reaction, in kj/mol for several different reactions,. This experiment has three primary objectives: For example, when an exothermic. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Measurement of an enthalpy change. A calorimeter is a device used to determine heat flow during a chemical or physical. Graph Of Calorimetry.
From www.researchgate.net
Differential scanning calorimetry (DSC) curve of starch extracted from Graph Of Calorimetry Determine the hrxn, the enthalpy of reaction, in kj/mol for several different reactions,. Apply the first law of thermodynamics to calorimetry. Find the heat capacity (cp) of a calorimeter and contents (calibration). Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. A doubled styrofoam cup fitted with a cover in which. Graph Of Calorimetry.
From www.researchgate.net
Differential scanning calorimetry (DSC) curves for plain epoxy resin at Graph Of Calorimetry This experiment has three primary objectives: Apply the first law of thermodynamics to calorimetry. Calorimetry is the measurement enthalpy changes in chemical reactions. A calorimeter is a device used to determine heat flow during a chemical or physical change. For example, when an exothermic. A doubled styrofoam cup fitted with a cover in which a hole. A calorimeter is a. Graph Of Calorimetry.
From www.youtube.com
CALORIMETRY Heating Curve of ICE (PHASE CHANGES GRAPH ) PPV Part 2 Graph Of Calorimetry Measurement of an enthalpy change. Determine the hrxn, the enthalpy of reaction, in kj/mol for several different reactions,. Calculating the enthalpy change of reaction, hrfrom experimental data. A doubled styrofoam cup fitted with a cover in which a hole. There are two types of calorimetry experiments you need to know:. This experiment has three primary objectives: Calorimetry is the measurement. Graph Of Calorimetry.
From www.researchgate.net
Differential Scanning Calorimetry Graph of (a) Theophylline Graph Of Calorimetry Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Find the heat capacity (cp) of a calorimeter and contents (calibration). There are two types of calorimetry experiments you need to know:. This experiment has three primary objectives: Calculating the enthalpy change of reaction, hrfrom experimental data. Measurement of an enthalpy change. For example,. Graph Of Calorimetry.
From www.researchgate.net
Calorimetric data. (a) Heat flow calorimetry curves and (b) Cumulative Graph Of Calorimetry Calorimetry is the measurement enthalpy changes in chemical reactions. Apply the first law of thermodynamics to calorimetry. For example, when an exothermic. A calorimeter is a device used to determine heat flow during a chemical or physical change. Calculating the enthalpy change of reaction, hrfrom experimental data. Determine the hrxn, the enthalpy of reaction, in kj/mol for several different reactions,.. Graph Of Calorimetry.
From www.researchgate.net
Differential scanning calorimetry (DSC) curves (first derivative) of Graph Of Calorimetry Determine the hrxn, the enthalpy of reaction, in kj/mol for several different reactions,. A calorimeter is a device used to determine heat flow during a chemical or physical change. Calorimetry is the measurement enthalpy changes in chemical reactions. There are two types of calorimetry experiments you need to know:. Calculating the enthalpy change of reaction, hrfrom experimental data. Use our. Graph Of Calorimetry.
From wisc.pb.unizin.org
Calorimetry continued Phase Changes and Heating Curves (M6Q6) UW Graph Of Calorimetry Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. A doubled styrofoam cup fitted with a cover in which a hole. There are two types of calorimetry experiments you need to know:. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Graph Of Calorimetry.
From www.researchgate.net
Isothermal titration calorimetry data obtained for the reaction of Graph Of Calorimetry A calorimeter is a device used to determine heat flow during a chemical or physical change. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. Determine the hrxn, the enthalpy of reaction, in kj/mol for several different reactions,. Calculate heat, temperature change, and specific heat after thermal. Graph Of Calorimetry.
From www.researchgate.net
Exothermic curves from differential scanning calorimetry (DSC) analysis Graph Of Calorimetry Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. Measurement of an enthalpy change. Apply the first law of thermodynamics to calorimetry. Calorimetry is the measurement enthalpy changes in chemical reactions. Find the heat capacity (cp) of a calorimeter and contents (calibration). A calorimeter is a device. Graph Of Calorimetry.
From stock.adobe.com
Vettoriale Stock DSC curve isolated on white. Differential scanning Graph Of Calorimetry A calorimeter is a device used to determine heat flow during a chemical or physical change. Calorimetry is the measurement enthalpy changes in chemical reactions. For example, when an exothermic. Calculating the enthalpy change of reaction, hrfrom experimental data. Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus. Graph Of Calorimetry.
From www.researchgate.net
Differential Scanning Calorimetry (DSC) thermograms of powders PA2200 Graph Of Calorimetry Find the heat capacity (cp) of a calorimeter and contents (calibration). This experiment has three primary objectives: Apply the first law of thermodynamics to calorimetry. A doubled styrofoam cup fitted with a cover in which a hole. Measurement of an enthalpy change. A calorimeter is a device used to determine heat flow during a chemical or physical change. Determine the. Graph Of Calorimetry.
From sites.google.com
Calorimetry Preliminary HSC Chemistry Graph Of Calorimetry A doubled styrofoam cup fitted with a cover in which a hole. Find the heat capacity (cp) of a calorimeter and contents (calibration). Calculating the enthalpy change of reaction, hrfrom experimental data. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the measurement enthalpy changes in chemical reactions.. Graph Of Calorimetry.
From www.researchgate.net
Exemplary Differential scanning calorimetry curves — First heating Graph Of Calorimetry Apply the first law of thermodynamics to calorimetry. Determine the hrxn, the enthalpy of reaction, in kj/mol for several different reactions,. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific. Graph Of Calorimetry.
From www.researchgate.net
The temperaturevstime graph for the of H 2 O 2 (aq Graph Of Calorimetry Find the heat capacity (cp) of a calorimeter and contents (calibration). Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. For example, when an exothermic. A doubled styrofoam cup fitted with a cover in which a hole. There are two types of calorimetry experiments you need to know:. This experiment has. Graph Of Calorimetry.
From www.youtube.com
BTec Calorimetry graph calculating a gradient YouTube Graph Of Calorimetry Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. A calorimeter is a device used to determine heat flow during a chemical or physical change. This experiment has three primary objectives: Calculating the enthalpy change of reaction, hrfrom experimental data. Find the heat capacity (cp) of a calorimeter and contents (calibration). Apply the. Graph Of Calorimetry.
From www.researchgate.net
Differential scanning calorimetry graph depicting different melting Graph Of Calorimetry A calorimeter is a device used to determine heat flow during a chemical or physical change. Apply the first law of thermodynamics to calorimetry. For example, when an exothermic. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. Find the heat capacity (cp) of a calorimeter and. Graph Of Calorimetry.
From www.researchgate.net
Differential scanning calorimetry (DSC) thermograms of (a Graph Of Calorimetry Calorimetry is the measurement enthalpy changes in chemical reactions. There are two types of calorimetry experiments you need to know:. A doubled styrofoam cup fitted with a cover in which a hole. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. Compare heat flow from hot to. Graph Of Calorimetry.
From www.researchgate.net
Differential scanning calorimetry graph of heat flow(W/g) vs Graph Of Calorimetry There are two types of calorimetry experiments you need to know:. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. Calorimetry is the measurement enthalpy changes in chemical. Graph Of Calorimetry.
From www.mooreanalytical.com
Thermal Analysis TGA/DSC MooreAnalytical Graph Of Calorimetry A doubled styrofoam cup fitted with a cover in which a hole. A calorimeter is a device used to determine heat flow during a chemical or physical change. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. A calorimeter is a device used to measure the amount of heat involved in. Graph Of Calorimetry.
From es.scribd.com
Calorimetry Graphs PDF Graph Of Calorimetry Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Find the heat capacity (cp) of a calorimeter and contents (calibration). Calculating the enthalpy change of reaction, hrfrom experimental data. Apply the first law of thermodynamics to calorimetry. For example, when an exothermic. Calorimetry is the measurement enthalpy changes in chemical reactions.. Graph Of Calorimetry.
From dxoazusse.blob.core.windows.net
Food Calorimetry Equation at Jesse Freeman blog Graph Of Calorimetry A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. A calorimeter is a device used to determine heat flow during a chemical or physical change. Apply the first law of thermodynamics to calorimetry. This. Graph Of Calorimetry.
From www.youtube.com
Bomb Calorimetry Introduction Physical Chemistry Laboratory YouTube Graph Of Calorimetry A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. This experiment has three primary objectives: Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. A. Graph Of Calorimetry.
From mavink.com
Differential Scanning Calorimetry Curve Graph Of Calorimetry Calorimetry is the measurement enthalpy changes in chemical reactions. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. For example, when an exothermic. Find the heat capacity (cp) of a calorimeter and contents (calibration). Measurement of an enthalpy change. Compare heat flow from hot to cold objects in an ideal calorimeter. Graph Of Calorimetry.
From www.researchgate.net
Differential scanning calorimetry (DSC) curves for PEEK and PEEK Graph Of Calorimetry Calorimetry is the measurement enthalpy changes in chemical reactions. Determine the hrxn, the enthalpy of reaction, in kj/mol for several different reactions,. For example, when an exothermic. Measurement of an enthalpy change. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Find the heat capacity (cp) of a calorimeter and contents (calibration). Use. Graph Of Calorimetry.
From www.surfacesciencewestern.com
Differential Scanning Calorimetry (DSC) Surface Science Western Graph Of Calorimetry Find the heat capacity (cp) of a calorimeter and contents (calibration). Calorimetry is the measurement enthalpy changes in chemical reactions. A calorimeter is a device used to determine heat flow during a chemical or physical change. A doubled styrofoam cup fitted with a cover in which a hole. This experiment has three primary objectives: Measurement of an enthalpy change. Compare. Graph Of Calorimetry.
From www.huck.psu.edu
Differential Scanning Calorimetry Automated Calorimetry Facility Graph Of Calorimetry Find the heat capacity (cp) of a calorimeter and contents (calibration). A calorimeter is a device used to determine heat flow during a chemical or physical change. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. There are two types of calorimetry experiments you need to know:.. Graph Of Calorimetry.