Titration Reaction Equivalence Point . In a titration, it is. The equivalence point of a titration. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. By the end of this section, you will be able to: At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. In other words, while titrating, it. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution.
from www.chegg.com
By the end of this section, you will be able to: The equivalence point of a titration. In other words, while titrating, it. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. In a titration, it is. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base.
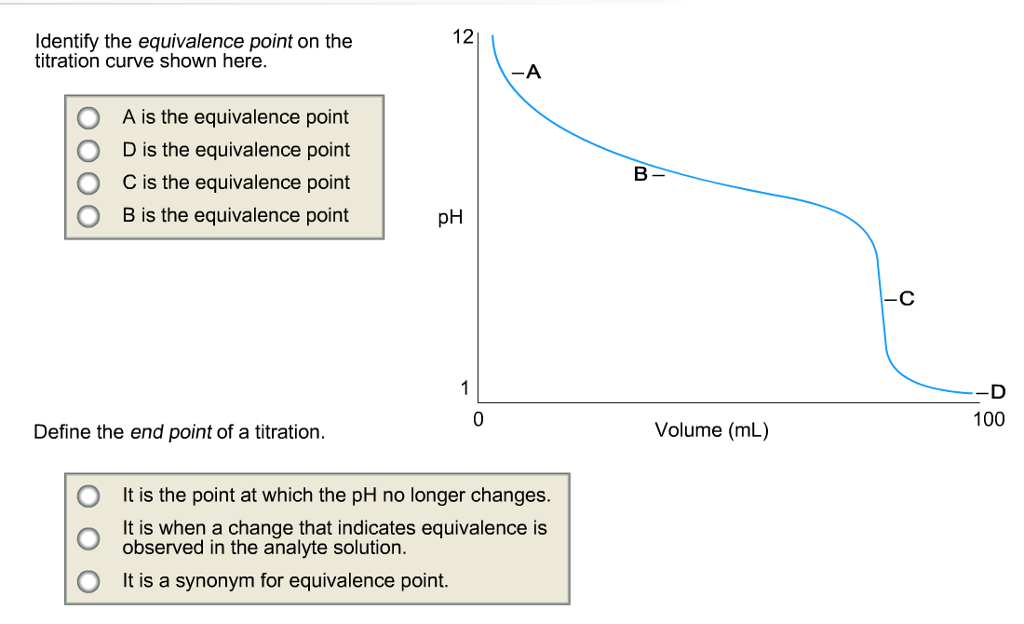
Solved Identify the equivalence point on the titration curve
Titration Reaction Equivalence Point At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. By the end of this section, you will be able to: At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. The equivalence point of a titration. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. In other words, while titrating, it. In a titration, it is.
From schoolbag.info
What volume of NaOH( aq ) would be needed to reach the equivalence Titration Reaction Equivalence Point By the end of this section, you will be able to: The equivalence point of a titration. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. A titration is a volumetric technique in which a solution of one reactant (the titrant) is. Titration Reaction Equivalence Point.
From dxoeyowlb.blob.core.windows.net
Equivalence Point Base Titration at Myrna Sanchez blog Titration Reaction Equivalence Point A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. The. Titration Reaction Equivalence Point.
From philschatz.com
AcidBase Titrations · Chemistry Titration Reaction Equivalence Point The equivalence point of a titration. In other words, while titrating, it. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. In a titration, it is. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the. Titration Reaction Equivalence Point.
From www.pearson.com
The following pictures represent solutions at various stages in t Titration Reaction Equivalence Point The equivalence point of a titration. By the end of this section, you will be able to: In a titration, it is. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration. Titration Reaction Equivalence Point.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Titration Reaction Equivalence Point In other words, while titrating, it. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. A titration. Titration Reaction Equivalence Point.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Reaction Equivalence Point At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. The equivalence point or stoichiometric point is the point in a chemical reaction when there. Titration Reaction Equivalence Point.
From www.youtube.com
Strong acid / strong base titration pH at equivalence point YouTube Titration Reaction Equivalence Point At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. In other words, while titrating, it. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. In a titration, it is. The equivalence point of a chemical. Titration Reaction Equivalence Point.
From pdfslide.net
(PPT) Acid Base Titrations EQUIVALENCE POINT The equivalence point Titration Reaction Equivalence Point The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. The equivalence point of a titration. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. At the. Titration Reaction Equivalence Point.
From saylordotorg.github.io
AcidBase Titrations Titration Reaction Equivalence Point The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to. Titration Reaction Equivalence Point.
From solvedlib.com
What is a titration? What is the equivalence point o… SolvedLib Titration Reaction Equivalence Point A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. In a titration, it is. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. Titration Reaction Equivalence Point.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 Titration Reaction Equivalence Point In other words, while titrating, it. By the end of this section, you will be able to: In a titration, it is. The equivalence point of a titration. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached.. Titration Reaction Equivalence Point.
From www.youtube.com
AcidBase Titration Equivalence Point YouTube Titration Reaction Equivalence Point Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. In other words, while titrating, it. The equivalence point of a titration. In a titration, it is. The equivalence point of a chemical reaction is the point at which equal quantities of reactants. Titration Reaction Equivalence Point.
From www.youtube.com
Titration Weak base/Strong acid Equivalence Point YouTube Titration Reaction Equivalence Point A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. The equivalence point of a titration. By the end. Titration Reaction Equivalence Point.
From goodttorials.blogspot.com
How To Find Equivalence Point From Titration Data Titration Reaction Equivalence Point The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. In a titration, it is. The equivalence point or stoichiometric point is the point. Titration Reaction Equivalence Point.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Titration Reaction Equivalence Point The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. By the end of this section, you will be able to: At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. A titration is a volumetric technique in which a solution of one. Titration Reaction Equivalence Point.
From study.com
Finding the Equivalence Point Titration & Examples Lesson Titration Reaction Equivalence Point By the end of this section, you will be able to: A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a. Titration Reaction Equivalence Point.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Titration Reaction Equivalence Point Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. The equivalence point of a titration. By the end of this section, you will be able to: In a titration, it is. At the equivalence point in a neutralization, the moles of acid. Titration Reaction Equivalence Point.
From www.chemistrystudent.com
Finding Ka using a Titration Curve (A2level) ChemistryStudent Titration Reaction Equivalence Point The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. The equivalence point of a titration. A titration is a volumetric technique in which. Titration Reaction Equivalence Point.
From www.youtube.com
Titration Weak Acid Strong Base Equivalence Point YouTube Titration Reaction Equivalence Point In a titration, it is. In other words, while titrating, it. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. By the end of this section, you will be able to: A titration is a volumetric technique in which a solution of. Titration Reaction Equivalence Point.
From psiberg.com
The Equivalence Point Acid/Base Titrations PSIBERG Titration Reaction Equivalence Point In a titration, it is. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. In other words,. Titration Reaction Equivalence Point.
From www.youtube.com
Titration Curves, Equivalence Point YouTube Titration Reaction Equivalence Point A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The equivalence point of a titration. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The equivalence point of. Titration Reaction Equivalence Point.
From mungfali.com
Equivalence Points On Titration Graph Titration Reaction Equivalence Point In a titration, it is. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. The equivalence point of a titration. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. In other words, while titrating, it.. Titration Reaction Equivalence Point.
From www.youtube.com
Titrations and the M1V1=M2V2 Math at The Equivalence Point YouTube Titration Reaction Equivalence Point A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The equivalence point of a titration. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to. Titration Reaction Equivalence Point.
From www.youtube.com
Weak acid / strong base titration pH at equivalence point YouTube Titration Reaction Equivalence Point The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. In other words, while titrating, it. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. By the end of this section, you will. Titration Reaction Equivalence Point.
From www.chegg.com
Solved Identify the equivalence point on the titration curve Titration Reaction Equivalence Point A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. The equivalence point or stoichiometric point is the point. Titration Reaction Equivalence Point.
From chem.libretexts.org
Redox Titration Chemistry LibreTexts Titration Reaction Equivalence Point The equivalence point of a titration. In a titration, it is. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to. Titration Reaction Equivalence Point.
From www.numerade.com
SOLVED Consider the titration curve below for the titration of oxalic Titration Reaction Equivalence Point A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. The. Titration Reaction Equivalence Point.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Titration Reaction Equivalence Point In a titration, it is. In other words, while titrating, it. By the end of this section, you will be able to: Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. The equivalence point of a chemical reaction is the point at. Titration Reaction Equivalence Point.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration Reaction Equivalence Point At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. By the end of this section, you will be able to: In a titration, it is. The equivalence point of a titration. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution. Titration Reaction Equivalence Point.
From www.pinterest.ph
Equivalence Point definition The point in a chemical reaction at which Titration Reaction Equivalence Point At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The equivalence point of a titration. In a titration, it is. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Titration Reaction Equivalence Point.
From dxoeyowlb.blob.core.windows.net
Equivalence Point Base Titration at Myrna Sanchez blog Titration Reaction Equivalence Point In other words, while titrating, it. The equivalence point of a titration. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. By the end of this section, you will be able to:. Titration Reaction Equivalence Point.
From www.showme.com
Titration Curve Explained Science, Chemistry ShowMe Titration Reaction Equivalence Point In a titration, it is. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. By the end of this section, you will be able to: The equivalence point of a titration. The equivalence point or stoichiometric point. Titration Reaction Equivalence Point.
From chemistrytalk.org
Titration Curves & Equivalence Point Calculations ChemTalk Titration Reaction Equivalence Point In a titration, it is. The equivalence point of a titration. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are. Titration Reaction Equivalence Point.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Reaction Equivalence Point A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. In other words, while titrating, it. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. In a titration, it. Titration Reaction Equivalence Point.
From chemistryguru.com.sg
Titration Curve for Weak Acid Strong Base Titration Reaction Equivalence Point The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. In other words, while titrating, it. In a titration, it is. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point. Titration Reaction Equivalence Point.