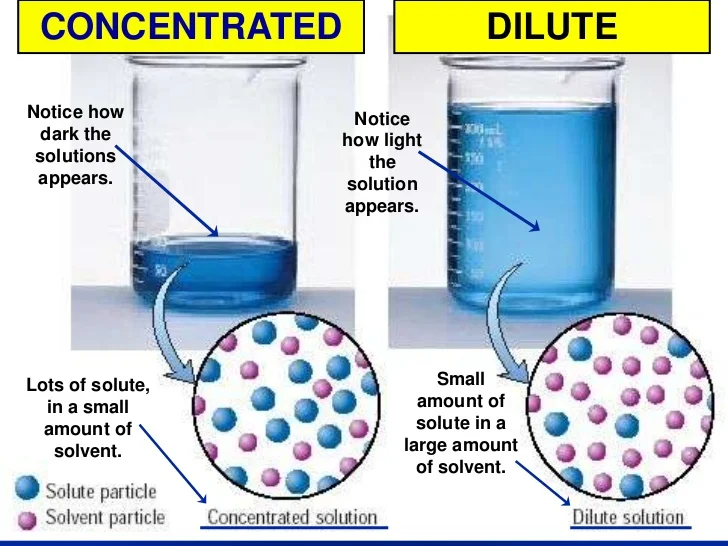
Dilute Quantity . We will begin our discussion of. The diluted liquid needs to be thoroughly mixed to achieve. 9.5 dilutions and concentrations. dilution of solutions. state whether the concentration of a solution is directly or indirectly proportional to its volume. to dilute solutions, such as simple household solutions, make sure you. the dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). Learn how to dilute and concentrate solutions. Dilution is a process used to lower the concentration of the original solution by adding more solvent. we are often concerned with how much solute is dissolved in a given amount of solution.
from www.slideshare.net
9.5 dilutions and concentrations. we are often concerned with how much solute is dissolved in a given amount of solution. The diluted liquid needs to be thoroughly mixed to achieve. Learn how to dilute and concentrate solutions. state whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is a process used to lower the concentration of the original solution by adding more solvent. to dilute solutions, such as simple household solutions, make sure you. We will begin our discussion of. dilution of solutions. the dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water).
Chapter 16 solutions
Dilute Quantity We will begin our discussion of. Dilution is a process used to lower the concentration of the original solution by adding more solvent. 9.5 dilutions and concentrations. dilution of solutions. Learn how to dilute and concentrate solutions. We will begin our discussion of. the dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). to dilute solutions, such as simple household solutions, make sure you. we are often concerned with how much solute is dissolved in a given amount of solution. state whether the concentration of a solution is directly or indirectly proportional to its volume. The diluted liquid needs to be thoroughly mixed to achieve.
From slideplayer.com
Acids & Bases Introduction ppt download Dilute Quantity 9.5 dilutions and concentrations. state whether the concentration of a solution is directly or indirectly proportional to its volume. We will begin our discussion of. to dilute solutions, such as simple household solutions, make sure you. Dilution is a process used to lower the concentration of the original solution by adding more solvent. we are often. Dilute Quantity.
From www.numerade.com
SOLVED A 4 kg sample of aqueous ethanol solution is 76 wt ethanol Dilute Quantity Dilution is a process used to lower the concentration of the original solution by adding more solvent. Learn how to dilute and concentrate solutions. 9.5 dilutions and concentrations. We will begin our discussion of. The diluted liquid needs to be thoroughly mixed to achieve. dilution of solutions. we are often concerned with how much solute is dissolved. Dilute Quantity.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube Dilute Quantity the dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). We will begin our discussion of. Learn how to dilute and concentrate solutions. The diluted liquid needs to be thoroughly mixed to achieve. we are often concerned with how much solute is dissolved in a given amount of solution.. Dilute Quantity.
From testbook.com
Dilute Acid Learn Definition, Examples, Formula and Properties Dilute Quantity to dilute solutions, such as simple household solutions, make sure you. 9.5 dilutions and concentrations. dilution of solutions. We will begin our discussion of. Learn how to dilute and concentrate solutions. state whether the concentration of a solution is directly or indirectly proportional to its volume. The diluted liquid needs to be thoroughly mixed to achieve.. Dilute Quantity.
From slideplayer.com
Solutions Chapter 15 Chapter ppt download Dilute Quantity the dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). to dilute solutions, such as simple household solutions, make sure you. we are often concerned with how much solute is dissolved in a given amount of solution. 9.5 dilutions and concentrations. state whether the concentration of. Dilute Quantity.
From www.youtube.com
What is the difference between a concentrated solution and a dilute Dilute Quantity dilution of solutions. 9.5 dilutions and concentrations. we are often concerned with how much solute is dissolved in a given amount of solution. state whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is a process used to lower the concentration of the original solution by adding more solvent. We. Dilute Quantity.
From chem.libretexts.org
5.2 Solutions and Dilutions Chemistry LibreTexts Dilute Quantity 9.5 dilutions and concentrations. we are often concerned with how much solute is dissolved in a given amount of solution. to dilute solutions, such as simple household solutions, make sure you. Dilution is a process used to lower the concentration of the original solution by adding more solvent. The diluted liquid needs to be thoroughly mixed to. Dilute Quantity.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilute Quantity 9.5 dilutions and concentrations. the dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). to dilute solutions, such as simple household solutions, make sure you. We will begin our discussion of. The diluted liquid needs to be thoroughly mixed to achieve. dilution of solutions. state whether. Dilute Quantity.
From onelab.andrewalliance.com
Dilute and Shoot Protocol OneLab Dilute Quantity to dilute solutions, such as simple household solutions, make sure you. 9.5 dilutions and concentrations. the dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). dilution of solutions. The diluted liquid needs to be thoroughly mixed to achieve. state whether the concentration of a solution is. Dilute Quantity.
From www.numerade.com
SOLVEDWhat quantity remains constant when you dilute a solution? Dilute Quantity the dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). 9.5 dilutions and concentrations. dilution of solutions. Dilution is a process used to lower the concentration of the original solution by adding more solvent. We will begin our discussion of. we are often concerned with how much. Dilute Quantity.
From www.scribd.com
Dilute solutions PDF Materials Quantity Dilute Quantity 9.5 dilutions and concentrations. we are often concerned with how much solute is dissolved in a given amount of solution. to dilute solutions, such as simple household solutions, make sure you. The diluted liquid needs to be thoroughly mixed to achieve. Learn how to dilute and concentrate solutions. Dilution is a process used to lower the concentration. Dilute Quantity.
From www.youtube.com
How To Dilute Chemicals Dilution Ratios Explained! YouTube Dilute Quantity state whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is a process used to lower the concentration of the original solution by adding more solvent. The diluted liquid needs to be thoroughly mixed to achieve. dilution of solutions. the dilution ratio is the ratio of the solute (the substance to. Dilute Quantity.
From www.youtube.com
Does quantity dilute kindness? YouTube Dilute Quantity Learn how to dilute and concentrate solutions. we are often concerned with how much solute is dissolved in a given amount of solution. Dilution is a process used to lower the concentration of the original solution by adding more solvent. We will begin our discussion of. The diluted liquid needs to be thoroughly mixed to achieve. the dilution. Dilute Quantity.
From www.slideserve.com
PPT Grade 7 Science PowerPoint Presentation, free download ID2229802 Dilute Quantity Dilution is a process used to lower the concentration of the original solution by adding more solvent. the dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). 9.5 dilutions and concentrations. Learn how to dilute and concentrate solutions. We will begin our discussion of. The diluted liquid needs to. Dilute Quantity.
From byjus.com
What are the major differences between dilute and concentrated acids? Dilute Quantity We will begin our discussion of. to dilute solutions, such as simple household solutions, make sure you. Dilution is a process used to lower the concentration of the original solution by adding more solvent. we are often concerned with how much solute is dissolved in a given amount of solution. the dilution ratio is the ratio of. Dilute Quantity.
From www.slideserve.com
PPT Grade 7 Science PowerPoint Presentation, free download ID2229802 Dilute Quantity The diluted liquid needs to be thoroughly mixed to achieve. We will begin our discussion of. 9.5 dilutions and concentrations. dilution of solutions. the dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). state whether the concentration of a solution is directly or indirectly proportional to its. Dilute Quantity.
From tms-lab.com
Automatic dilutor and plater, easySpiral Dilute Team Medical Dilute Quantity we are often concerned with how much solute is dissolved in a given amount of solution. state whether the concentration of a solution is directly or indirectly proportional to its volume. to dilute solutions, such as simple household solutions, make sure you. dilution of solutions. We will begin our discussion of. Dilution is a process used. Dilute Quantity.
From slideplayer.com
A dilute solution is one that contains a small amount of solute. ppt Dilute Quantity The diluted liquid needs to be thoroughly mixed to achieve. to dilute solutions, such as simple household solutions, make sure you. we are often concerned with how much solute is dissolved in a given amount of solution. Dilution is a process used to lower the concentration of the original solution by adding more solvent. the dilution ratio. Dilute Quantity.
From www.scribd.com
Cds How To Dilute PDF Parts Per Notation Quantity Dilute Quantity to dilute solutions, such as simple household solutions, make sure you. The diluted liquid needs to be thoroughly mixed to achieve. Dilution is a process used to lower the concentration of the original solution by adding more solvent. the dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). Learn. Dilute Quantity.
From slideplayer.com
Solutions and Solubility ppt download Dilute Quantity Dilution is a process used to lower the concentration of the original solution by adding more solvent. 9.5 dilutions and concentrations. state whether the concentration of a solution is directly or indirectly proportional to its volume. dilution of solutions. We will begin our discussion of. we are often concerned with how much solute is dissolved in. Dilute Quantity.
From borenew.weebly.com
Serial Dilution Calculation Examples borenew Dilute Quantity Learn how to dilute and concentrate solutions. the dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). state whether the concentration of a solution is directly or indirectly proportional to its volume. we are often concerned with how much solute is dissolved in a given amount of solution.. Dilute Quantity.
From slideplayer.com
Chapter 8 Some substances dissolve to form solutions faster and more Dilute Quantity We will begin our discussion of. state whether the concentration of a solution is directly or indirectly proportional to its volume. Learn how to dilute and concentrate solutions. we are often concerned with how much solute is dissolved in a given amount of solution. dilution of solutions. The diluted liquid needs to be thoroughly mixed to achieve.. Dilute Quantity.
From www.youtube.com
Dilute, Concentrated and saturated solution YouTube Dilute Quantity the dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). state whether the concentration of a solution is directly or indirectly proportional to its volume. 9.5 dilutions and concentrations. Dilution is a process used to lower the concentration of the original solution by adding more solvent. we. Dilute Quantity.
From www.slideserve.com
PPT Grade 7 Science PowerPoint Presentation, free download ID2229802 Dilute Quantity 9.5 dilutions and concentrations. to dilute solutions, such as simple household solutions, make sure you. we are often concerned with how much solute is dissolved in a given amount of solution. state whether the concentration of a solution is directly or indirectly proportional to its volume. The diluted liquid needs to be thoroughly mixed to achieve.. Dilute Quantity.
From www.wikihow.com
How to Dilute an Acid (with Pictures) wikiHow Dilute Quantity Learn how to dilute and concentrate solutions. the dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). Dilution is a process used to lower the concentration of the original solution by adding more solvent. 9.5 dilutions and concentrations. dilution of solutions. We will begin our discussion of. . Dilute Quantity.
From www.thoughtco.com
What Is the Definition of Dilute? Dilute Quantity we are often concerned with how much solute is dissolved in a given amount of solution. Dilution is a process used to lower the concentration of the original solution by adding more solvent. to dilute solutions, such as simple household solutions, make sure you. Learn how to dilute and concentrate solutions. 9.5 dilutions and concentrations. The diluted. Dilute Quantity.
From ar.inspiredpencil.com
Concentrated Vs Dilute Solutions Dilute Quantity dilution of solutions. We will begin our discussion of. Dilution is a process used to lower the concentration of the original solution by adding more solvent. The diluted liquid needs to be thoroughly mixed to achieve. 9.5 dilutions and concentrations. state whether the concentration of a solution is directly or indirectly proportional to its volume. we. Dilute Quantity.
From slideplayer.com
Solution. ppt download Dilute Quantity we are often concerned with how much solute is dissolved in a given amount of solution. dilution of solutions. to dilute solutions, such as simple household solutions, make sure you. the dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). state whether the concentration of a. Dilute Quantity.
From ar.inspiredpencil.com
Concentrated Vs Dilute Solutions Dilute Quantity we are often concerned with how much solute is dissolved in a given amount of solution. Learn how to dilute and concentrate solutions. state whether the concentration of a solution is directly or indirectly proportional to its volume. The diluted liquid needs to be thoroughly mixed to achieve. dilution of solutions. the dilution ratio is the. Dilute Quantity.
From www.slideshare.net
Chapter 16 solutions Dilute Quantity We will begin our discussion of. 9.5 dilutions and concentrations. to dilute solutions, such as simple household solutions, make sure you. The diluted liquid needs to be thoroughly mixed to achieve. we are often concerned with how much solute is dissolved in a given amount of solution. Dilution is a process used to lower the concentration of. Dilute Quantity.
From slideplayer.com
Molarity and Dilutions ppt download Dilute Quantity to dilute solutions, such as simple household solutions, make sure you. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Learn how to dilute and concentrate solutions. we are often concerned with how much solute is dissolved in a given amount of solution. 9.5 dilutions and concentrations. We will. Dilute Quantity.
From www.researchgate.net
Procedure for measuring dilute solutions Download Scientific Diagram Dilute Quantity Dilution is a process used to lower the concentration of the original solution by adding more solvent. we are often concerned with how much solute is dissolved in a given amount of solution. dilution of solutions. to dilute solutions, such as simple household solutions, make sure you. state whether the concentration of a solution is directly. Dilute Quantity.
From www.toppr.com
The passage of a constant current through a solution of dilute H2SO4 Dilute Quantity we are often concerned with how much solute is dissolved in a given amount of solution. dilution of solutions. state whether the concentration of a solution is directly or indirectly proportional to its volume. 9.5 dilutions and concentrations. the dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent. Dilute Quantity.
From ar.inspiredpencil.com
Concentrated Vs Dilute Solutions Dilute Quantity Learn how to dilute and concentrate solutions. to dilute solutions, such as simple household solutions, make sure you. we are often concerned with how much solute is dissolved in a given amount of solution. dilution of solutions. 9.5 dilutions and concentrations. The diluted liquid needs to be thoroughly mixed to achieve. state whether the concentration. Dilute Quantity.
From www.wolframalpha.com
Dilution Calculator WolframAlpha Chemistry Solvers Dilute Quantity the dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). 9.5 dilutions and concentrations. Learn how to dilute and concentrate solutions. The diluted liquid needs to be thoroughly mixed to achieve. state whether the concentration of a solution is directly or indirectly proportional to its volume. dilution. Dilute Quantity.