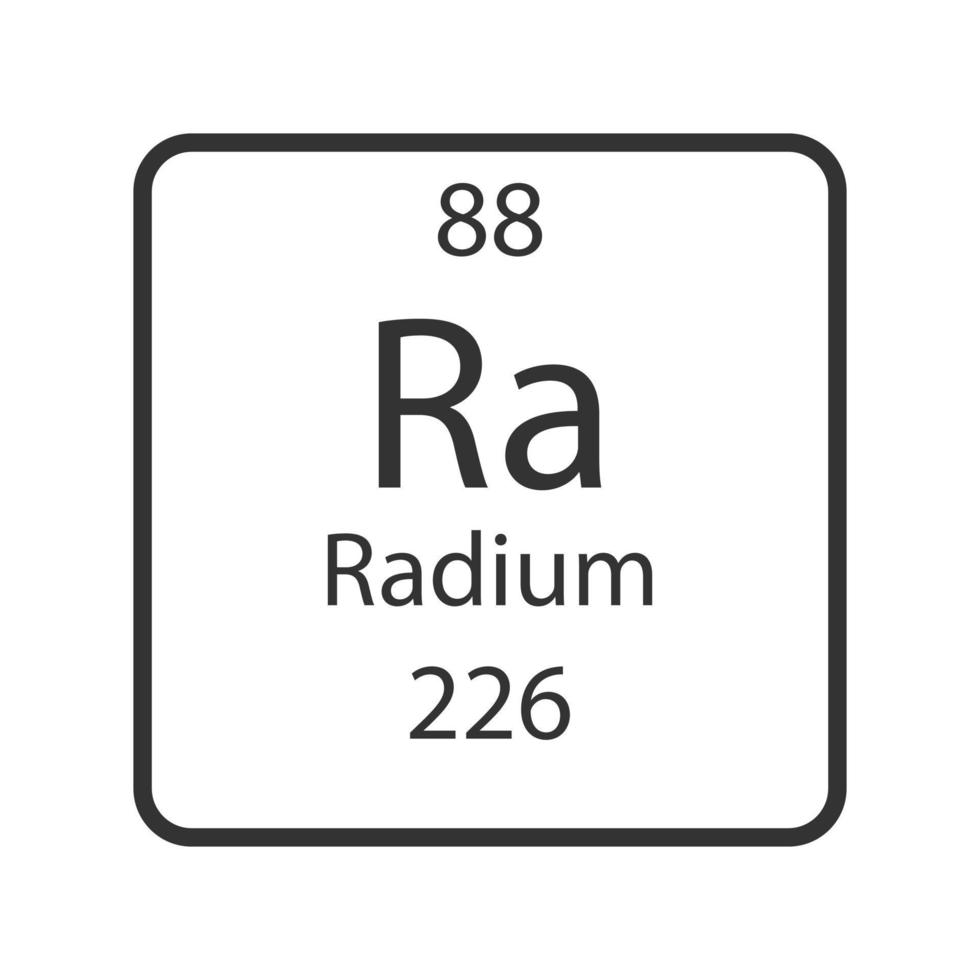
Characteristics Of Radium . It is created when uranium and thorium radioactively decay in the environment. Radium is approximately 2.7 million times more radioactive compared to uranium. Blockelements are organised into blocks by the orbital type in which. The element decomposes in water. Radium is a chemical element with the symbol ra and atomic number 88. Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated to be 5, and valence of 2. Pure radium metal is bright white when freshly prepared, although it blackens upon exposure to air. Its atomic symbol ra, atomic number 88,. Radium is an alkaline earth metal. Radium glows in the dark due to its intense radioactivity, which it imparts to other substances. Radium is silvery, lustrous, soft and radioactive element. The atomic number of each element increases by one, reading from left to right. Radium is a radioactive element of the alkaline earth series of metals. It is the sixth element in group 2 of the periodic table, also known as the.
from www.vecteezy.com
It is created when uranium and thorium radioactively decay in the environment. The element decomposes in water. Radium is a chemical element with the symbol ra and atomic number 88. Radium is approximately 2.7 million times more radioactive compared to uranium. Radium is silvery, lustrous, soft and radioactive element. Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated to be 5, and valence of 2. It is the sixth element in group 2 of the periodic table, also known as the. Pure radium metal is bright white when freshly prepared, although it blackens upon exposure to air. Radium is a radioactive element of the alkaline earth series of metals. Radium is an alkaline earth metal.
Radium symbol. Chemical element of the periodic table. Vector
Characteristics Of Radium Radium is silvery, lustrous, soft and radioactive element. Radium is a chemical element with the symbol ra and atomic number 88. The element decomposes in water. Radium is approximately 2.7 million times more radioactive compared to uranium. Radium glows in the dark due to its intense radioactivity, which it imparts to other substances. Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated to be 5, and valence of 2. Radium is an alkaline earth metal. Pure radium metal is bright white when freshly prepared, although it blackens upon exposure to air. Its atomic symbol ra, atomic number 88,. It is the sixth element in group 2 of the periodic table, also known as the. Radium is silvery, lustrous, soft and radioactive element. Blockelements are organised into blocks by the orbital type in which. It is created when uranium and thorium radioactively decay in the environment. The atomic number of each element increases by one, reading from left to right. Radium is a radioactive element of the alkaline earth series of metals.
From www.nuclear-power.com
What is Radium Properties of Radium Element Symbol Ra nuclear Characteristics Of Radium Its atomic symbol ra, atomic number 88,. The atomic number of each element increases by one, reading from left to right. Radium is a chemical element with the symbol ra and atomic number 88. Radium is silvery, lustrous, soft and radioactive element. Radium is approximately 2.7 million times more radioactive compared to uranium. Blockelements are organised into blocks by the. Characteristics Of Radium.
From www.vecteezy.com
Radium symbol. Chemical element of the periodic table. Vector Characteristics Of Radium Radium is an alkaline earth metal. It is the sixth element in group 2 of the periodic table, also known as the. The element decomposes in water. Radium is a radioactive element of the alkaline earth series of metals. Radium glows in the dark due to its intense radioactivity, which it imparts to other substances. Blockelements are organised into blocks. Characteristics Of Radium.
From www.vecteezy.com
Radium symbol. Chemical element of the periodic table. Vector Characteristics Of Radium Radium is silvery, lustrous, soft and radioactive element. Radium is a chemical element with the symbol ra and atomic number 88. It is created when uranium and thorium radioactively decay in the environment. The atomic number of each element increases by one, reading from left to right. Radium is a radioactive element of the alkaline earth series of metals. Radium. Characteristics Of Radium.
From www.chemistrylearner.com
Radium Facts, Symbol, Discovery, Properties, Uses Characteristics Of Radium Radium is approximately 2.7 million times more radioactive compared to uranium. Radium is a radioactive element of the alkaline earth series of metals. The atomic number of each element increases by one, reading from left to right. Radium is silvery, lustrous, soft and radioactive element. Pure radium metal is bright white when freshly prepared, although it blackens upon exposure to. Characteristics Of Radium.
From www.alamy.com
Radium atom, with mass and energy levels. Vector illustration Stock Characteristics Of Radium Radium is a chemical element with the symbol ra and atomic number 88. The element decomposes in water. Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated to be 5, and valence of 2. Radium glows in the dark due to its intense radioactivity, which it imparts to other substances. Radium is an alkaline earth. Characteristics Of Radium.
From www.alamy.com
Symbol and electron diagram for Radium illustration Stock Vector Image Characteristics Of Radium Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated to be 5, and valence of 2. The element decomposes in water. It is the sixth element in group 2 of the periodic table, also known as the. Radium glows in the dark due to its intense radioactivity, which it imparts to other substances. Blockelements are. Characteristics Of Radium.
From stock.adobe.com
Ra Radium Element Information Facts, Properties, Trends, Uses and Characteristics Of Radium Radium is an alkaline earth metal. Blockelements are organised into blocks by the orbital type in which. Radium is silvery, lustrous, soft and radioactive element. Its atomic symbol ra, atomic number 88,. Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated to be 5, and valence of 2. Pure radium metal is bright white when. Characteristics Of Radium.
From www.slideserve.com
PPT Radium PowerPoint Presentation, free download ID2424142 Characteristics Of Radium Radium glows in the dark due to its intense radioactivity, which it imparts to other substances. The element decomposes in water. It is created when uranium and thorium radioactively decay in the environment. It is the sixth element in group 2 of the periodic table, also known as the. Radium is a chemical element with the symbol ra and atomic. Characteristics Of Radium.
From www.dreamstime.com
Infographic of the Element of Radium Stock Vector Illustration of Characteristics Of Radium The element decomposes in water. The atomic number of each element increases by one, reading from left to right. Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated to be 5, and valence of 2. It is created when uranium and thorium radioactively decay in the environment. Pure radium metal is bright white when freshly. Characteristics Of Radium.
From www.animalia-life.club
Radium On The Periodic Table Of Elements Characteristics Of Radium Radium glows in the dark due to its intense radioactivity, which it imparts to other substances. Pure radium metal is bright white when freshly prepared, although it blackens upon exposure to air. Radium is approximately 2.7 million times more radioactive compared to uranium. Its atomic symbol ra, atomic number 88,. It is created when uranium and thorium radioactively decay in. Characteristics Of Radium.
From www.sciencephoto.com
Radium, atomic structure Stock Image C018/3769 Science Photo Library Characteristics Of Radium Radium glows in the dark due to its intense radioactivity, which it imparts to other substances. Its atomic symbol ra, atomic number 88,. Blockelements are organised into blocks by the orbital type in which. Pure radium metal is bright white when freshly prepared, although it blackens upon exposure to air. It is the sixth element in group 2 of the. Characteristics Of Radium.
From www.chemistrylearner.com
Radium Facts, Symbol, Discovery, Properties, Uses Characteristics Of Radium Blockelements are organised into blocks by the orbital type in which. Radium is silvery, lustrous, soft and radioactive element. The element decomposes in water. Its atomic symbol ra, atomic number 88,. Radium is a radioactive element of the alkaline earth series of metals. Radium glows in the dark due to its intense radioactivity, which it imparts to other substances. Radium. Characteristics Of Radium.
From www.sliderbase.com
Properties of Radium Characteristics Of Radium Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated to be 5, and valence of 2. Radium is an alkaline earth metal. Blockelements are organised into blocks by the orbital type in which. The atomic number of each element increases by one, reading from left to right. Radium glows in the dark due to its. Characteristics Of Radium.
From www.vectorstock.com
Radium ra periodic table element Royalty Free Vector Image Characteristics Of Radium Radium is an alkaline earth metal. Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated to be 5, and valence of 2. Radium glows in the dark due to its intense radioactivity, which it imparts to other substances. Its atomic symbol ra, atomic number 88,. Radium is a radioactive element of the alkaline earth series. Characteristics Of Radium.
From www.americanelements.com
Radium (Ra) AMERICAN ELEMENTS Characteristics Of Radium The element decomposes in water. Radium is approximately 2.7 million times more radioactive compared to uranium. Radium is a chemical element with the symbol ra and atomic number 88. Radium glows in the dark due to its intense radioactivity, which it imparts to other substances. The atomic number of each element increases by one, reading from left to right. Pure. Characteristics Of Radium.
From periodictableguide.com
Radium (Ra) Periodic Table (Element Information & More) Characteristics Of Radium Radium is a chemical element with the symbol ra and atomic number 88. Radium is a radioactive element of the alkaline earth series of metals. Radium is approximately 2.7 million times more radioactive compared to uranium. Blockelements are organised into blocks by the orbital type in which. Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity. Characteristics Of Radium.
From www.dreamstime.com
Element of Radium stock vector. Illustration of atomic 82537168 Characteristics Of Radium The element decomposes in water. Radium is approximately 2.7 million times more radioactive compared to uranium. Radium is a chemical element with the symbol ra and atomic number 88. Radium glows in the dark due to its intense radioactivity, which it imparts to other substances. It is the sixth element in group 2 of the periodic table, also known as. Characteristics Of Radium.
From www.vectorstock.com
Chemical element radium Royalty Free Vector Image Characteristics Of Radium Radium is an alkaline earth metal. It is created when uranium and thorium radioactively decay in the environment. Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated to be 5, and valence of 2. The element decomposes in water. Its atomic symbol ra, atomic number 88,. Radium is silvery, lustrous, soft and radioactive element. It. Characteristics Of Radium.
From www.dreamstime.com
Element of Radium stock vector. Illustration of atomic 88614843 Characteristics Of Radium Blockelements are organised into blocks by the orbital type in which. Its atomic symbol ra, atomic number 88,. Pure radium metal is bright white when freshly prepared, although it blackens upon exposure to air. It is created when uranium and thorium radioactively decay in the environment. Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated. Characteristics Of Radium.
From www.alamy.com
Ra Radium, Periodic Table of the Elements, Shell Structure of Radium Characteristics Of Radium Radium is a chemical element with the symbol ra and atomic number 88. Radium is silvery, lustrous, soft and radioactive element. The atomic number of each element increases by one, reading from left to right. Radium is an alkaline earth metal. Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated to be 5, and valence. Characteristics Of Radium.
From www.dreamstime.com
Radium Form Periodic Table of Elements Stock Illustration Characteristics Of Radium Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated to be 5, and valence of 2. Blockelements are organised into blocks by the orbital type in which. Radium is silvery, lustrous, soft and radioactive element. The atomic number of each element increases by one, reading from left to right. Radium is a chemical element with. Characteristics Of Radium.
From www.pinterest.com
Kids learn about the element radium and its chemistry including atomic Characteristics Of Radium It is the sixth element in group 2 of the periodic table, also known as the. Its atomic symbol ra, atomic number 88,. The element decomposes in water. Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated to be 5, and valence of 2. It is created when uranium and thorium radioactively decay in the. Characteristics Of Radium.
From www.schoolmykids.com
Radium (Ra) Element Information, Facts, Properties, Uses Periodic Characteristics Of Radium Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated to be 5, and valence of 2. Radium is silvery, lustrous, soft and radioactive element. Radium glows in the dark due to its intense radioactivity, which it imparts to other substances. Blockelements are organised into blocks by the orbital type in which. Radium is a radioactive. Characteristics Of Radium.
From www.examples.com
Radium (Ra) Definition, Preparation, Properties, Uses, Compounds Characteristics Of Radium Radium is a chemical element with the symbol ra and atomic number 88. Radium is an alkaline earth metal. It is the sixth element in group 2 of the periodic table, also known as the. Radium glows in the dark due to its intense radioactivity, which it imparts to other substances. Its atomic symbol ra, atomic number 88,. Radium is. Characteristics Of Radium.
From www.livescience.com
Facts About Radium Live Science Characteristics Of Radium It is the sixth element in group 2 of the periodic table, also known as the. Radium is approximately 2.7 million times more radioactive compared to uranium. Radium is a chemical element with the symbol ra and atomic number 88. The atomic number of each element increases by one, reading from left to right. Its atomic symbol ra, atomic number. Characteristics Of Radium.
From www.slideserve.com
PPT Radium PowerPoint Presentation ID2424142 Characteristics Of Radium Radium glows in the dark due to its intense radioactivity, which it imparts to other substances. The element decomposes in water. Radium is a chemical element with the symbol ra and atomic number 88. It is the sixth element in group 2 of the periodic table, also known as the. Pure radium metal is bright white when freshly prepared, although. Characteristics Of Radium.
From www.dreamstime.com
Radium Ra Chemical Element Periodic Table Stock Illustration Characteristics Of Radium Pure radium metal is bright white when freshly prepared, although it blackens upon exposure to air. Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated to be 5, and valence of 2. Its atomic symbol ra, atomic number 88,. Radium glows in the dark due to its intense radioactivity, which it imparts to other substances.. Characteristics Of Radium.
From en.wikipedia.org
Radium Wikipedia Characteristics Of Radium The atomic number of each element increases by one, reading from left to right. The element decomposes in water. Radium is a radioactive element of the alkaline earth series of metals. Blockelements are organised into blocks by the orbital type in which. It is created when uranium and thorium radioactively decay in the environment. It is the sixth element in. Characteristics Of Radium.
From www.dreamstime.com
Infographic of the Element of Radium Stock Vector Illustration of Characteristics Of Radium Radium is silvery, lustrous, soft and radioactive element. Its atomic symbol ra, atomic number 88,. Radium is a radioactive element of the alkaline earth series of metals. The element decomposes in water. Radium is approximately 2.7 million times more radioactive compared to uranium. Blockelements are organised into blocks by the orbital type in which. Radium is a chemical element with. Characteristics Of Radium.
From utedzz.blogspot.com
Periodic Table Radium Element Symbol Periodic Table Timeline Characteristics Of Radium Radium is a radioactive element of the alkaline earth series of metals. Radium is a chemical element with the symbol ra and atomic number 88. Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated to be 5, and valence of 2. The element decomposes in water. The atomic number of each element increases by one,. Characteristics Of Radium.
From www.alamy.com
Radium chemical element, Sign with atomic number and atomic weight Characteristics Of Radium Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated to be 5, and valence of 2. The element decomposes in water. Radium is an alkaline earth metal. Radium is approximately 2.7 million times more radioactive compared to uranium. Blockelements are organised into blocks by the orbital type in which. Radium is a radioactive element of. Characteristics Of Radium.
From www.animalia-life.club
Radium On The Periodic Table Of Elements Characteristics Of Radium Its atomic symbol ra, atomic number 88,. It is the sixth element in group 2 of the periodic table, also known as the. Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated to be 5, and valence of 2. It is created when uranium and thorium radioactively decay in the environment. Radium is a radioactive. Characteristics Of Radium.
From mintteam861.weebly.com
Radium Atomic Number mintteam Characteristics Of Radium The atomic number of each element increases by one, reading from left to right. Pure radium metal is bright white when freshly prepared, although it blackens upon exposure to air. Its atomic symbol ra, atomic number 88,. It is created when uranium and thorium radioactively decay in the environment. Radium has a melting point of 700°c, boiling point of 1140°c,. Characteristics Of Radium.
From www.sciencephoto.com
Radium, atomic structure Stock Image C023/2594 Science Photo Library Characteristics Of Radium Radium is approximately 2.7 million times more radioactive compared to uranium. The element decomposes in water. The atomic number of each element increases by one, reading from left to right. Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated to be 5, and valence of 2. Pure radium metal is bright white when freshly prepared,. Characteristics Of Radium.
From www.dreamstime.com
Diagram Representation Of The Element Radium Stock Illustration Image Characteristics Of Radium Radium has a melting point of 700°c, boiling point of 1140°c, specific gravity estimated to be 5, and valence of 2. Blockelements are organised into blocks by the orbital type in which. Radium is a chemical element with the symbol ra and atomic number 88. Radium is approximately 2.7 million times more radioactive compared to uranium. It is created when. Characteristics Of Radium.