You Pour Water To Acid . This is because acid and water react in a vigorous exothermic reaction , releasing heat, sometimes boiling the liquid. When you add water to acid, the water will layer on top of the more densely concentrated acid or base. Slowly pour all the acid into the water. Obtain the correct amounts of deionized (di) water in one beaker and acid in another. This is due to the hydrogen bonding in water, which means a lot of energy is needed to make it. The extreme heat produced cause the solution to boil and project the upper layer out of the container. The reason this occurs is due to the large amount of energy released in the hydration. When you mix concentrated sulfuric acid and water, you pour the acid into a larger volume of water. You should always add acid to water, otherwise you will end up with an extremely exothermic reaction that can even boil the water and cause violent bumping that can result in acid splashing all over the place. When acid is poured into water, it flows down the flask and mixes much better, so no boiling occurs. When you mix acid with water, it's extremely important to add the acid to the water rather than the other way around. This is referred to as dissociative energy release. Mixing the chemicals the other way can present a lab safety hazard.
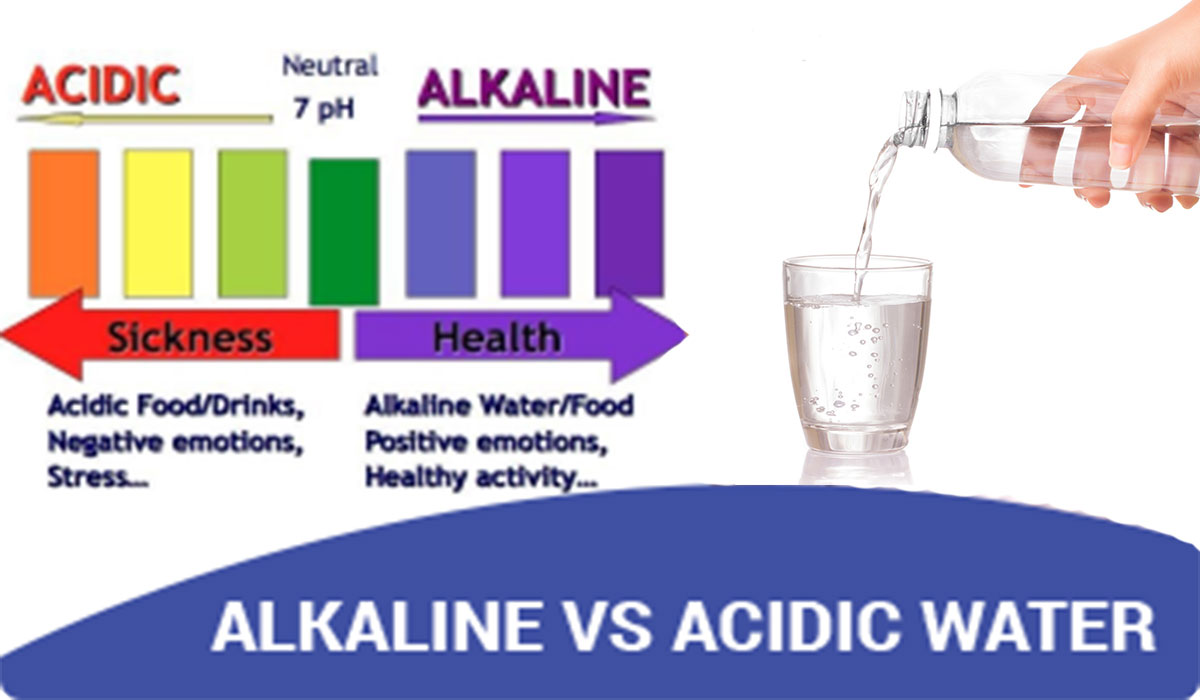
from netsolwater.com
Mixing the chemicals the other way can present a lab safety hazard. This is referred to as dissociative energy release. This is because acid and water react in a vigorous exothermic reaction , releasing heat, sometimes boiling the liquid. Slowly pour all the acid into the water. The reason this occurs is due to the large amount of energy released in the hydration. You should always add acid to water, otherwise you will end up with an extremely exothermic reaction that can even boil the water and cause violent bumping that can result in acid splashing all over the place. The extreme heat produced cause the solution to boil and project the upper layer out of the container. When acid is poured into water, it flows down the flask and mixes much better, so no boiling occurs. When you add water to acid, the water will layer on top of the more densely concentrated acid or base. When you mix concentrated sulfuric acid and water, you pour the acid into a larger volume of water.
What are alkaline water and acidic water? Netsol Water
You Pour Water To Acid Mixing the chemicals the other way can present a lab safety hazard. You should always add acid to water, otherwise you will end up with an extremely exothermic reaction that can even boil the water and cause violent bumping that can result in acid splashing all over the place. When you mix concentrated sulfuric acid and water, you pour the acid into a larger volume of water. Mixing the chemicals the other way can present a lab safety hazard. When you add water to acid, the water will layer on top of the more densely concentrated acid or base. The reason this occurs is due to the large amount of energy released in the hydration. The extreme heat produced cause the solution to boil and project the upper layer out of the container. Obtain the correct amounts of deionized (di) water in one beaker and acid in another. This is due to the hydrogen bonding in water, which means a lot of energy is needed to make it. Slowly pour all the acid into the water. When acid is poured into water, it flows down the flask and mixes much better, so no boiling occurs. This is referred to as dissociative energy release. When you mix acid with water, it's extremely important to add the acid to the water rather than the other way around. This is because acid and water react in a vigorous exothermic reaction , releasing heat, sometimes boiling the liquid.
From labpedia.net
Safety in the Clinical Laboratory You Pour Water To Acid When you mix concentrated sulfuric acid and water, you pour the acid into a larger volume of water. You should always add acid to water, otherwise you will end up with an extremely exothermic reaction that can even boil the water and cause violent bumping that can result in acid splashing all over the place. When you mix acid with. You Pour Water To Acid.
From sciencenotes.org
Add Acid to Water or Water to Acid? Safely Diluting Acids You Pour Water To Acid You should always add acid to water, otherwise you will end up with an extremely exothermic reaction that can even boil the water and cause violent bumping that can result in acid splashing all over the place. Obtain the correct amounts of deionized (di) water in one beaker and acid in another. When you add water to acid, the water. You Pour Water To Acid.
From www.youtube.com
Acid to water or water to acid !!! 🤔 YouTube You Pour Water To Acid Mixing the chemicals the other way can present a lab safety hazard. When you mix acid with water, it's extremely important to add the acid to the water rather than the other way around. The reason this occurs is due to the large amount of energy released in the hydration. This is due to the hydrogen bonding in water, which. You Pour Water To Acid.
From www.youtube.com
Acid and Water Experiments Acid in Water Or Water in Acid? YouTube You Pour Water To Acid The extreme heat produced cause the solution to boil and project the upper layer out of the container. This is because acid and water react in a vigorous exothermic reaction , releasing heat, sometimes boiling the liquid. Mixing the chemicals the other way can present a lab safety hazard. Slowly pour all the acid into the water. When you add. You Pour Water To Acid.
From www.youtube.com
What happens when you add water to Acid YouTube You Pour Water To Acid The reason this occurs is due to the large amount of energy released in the hydration. When you mix concentrated sulfuric acid and water, you pour the acid into a larger volume of water. This is because acid and water react in a vigorous exothermic reaction , releasing heat, sometimes boiling the liquid. When you add water to acid, the. You Pour Water To Acid.
From www.thoughtco.com
Do You Add Sulfuric Acid to Water or Vice Versa? You Pour Water To Acid When you add water to acid, the water will layer on top of the more densely concentrated acid or base. When you mix concentrated sulfuric acid and water, you pour the acid into a larger volume of water. When you mix acid with water, it's extremely important to add the acid to the water rather than the other way around.. You Pour Water To Acid.
From www.youtube.com
Adding Water to 98 Concentrated Sulphuric Acid YouTube You Pour Water To Acid The reason this occurs is due to the large amount of energy released in the hydration. Obtain the correct amounts of deionized (di) water in one beaker and acid in another. This is due to the hydrogen bonding in water, which means a lot of energy is needed to make it. This is referred to as dissociative energy release. Mixing. You Pour Water To Acid.
From slideplayer.com
Find 6 things that are unsafe ppt download You Pour Water To Acid The extreme heat produced cause the solution to boil and project the upper layer out of the container. This is because acid and water react in a vigorous exothermic reaction , releasing heat, sometimes boiling the liquid. When acid is poured into water, it flows down the flask and mixes much better, so no boiling occurs. When you mix concentrated. You Pour Water To Acid.
From www.youtube.com
Dilution of AcidWhy is it to add acid to water but not You Pour Water To Acid When you add water to acid, the water will layer on top of the more densely concentrated acid or base. The extreme heat produced cause the solution to boil and project the upper layer out of the container. Obtain the correct amounts of deionized (di) water in one beaker and acid in another. Mixing the chemicals the other way can. You Pour Water To Acid.
From www.teachoo.com
Assertion (A) To dilute sulphuric acid, acid is added to water and n You Pour Water To Acid This is referred to as dissociative energy release. This is due to the hydrogen bonding in water, which means a lot of energy is needed to make it. The reason this occurs is due to the large amount of energy released in the hydration. Mixing the chemicals the other way can present a lab safety hazard. This is because acid. You Pour Water To Acid.
From www.yaclass.in
Acid and base in a water solution — lesson. Science CBSE, Class 10. You Pour Water To Acid This is because acid and water react in a vigorous exothermic reaction , releasing heat, sometimes boiling the liquid. This is referred to as dissociative energy release. When you mix concentrated sulfuric acid and water, you pour the acid into a larger volume of water. You should always add acid to water, otherwise you will end up with an extremely. You Pour Water To Acid.
From www.youtube.com
Adding Water into Acid is Dangerous 😮 YouTube You Pour Water To Acid The reason this occurs is due to the large amount of energy released in the hydration. This is because acid and water react in a vigorous exothermic reaction , releasing heat, sometimes boiling the liquid. When you add water to acid, the water will layer on top of the more densely concentrated acid or base. You should always add acid. You Pour Water To Acid.
From www.youtube.com
What is Acidic Water and How Do You Treat It? YouTube You Pour Water To Acid When you mix concentrated sulfuric acid and water, you pour the acid into a larger volume of water. The reason this occurs is due to the large amount of energy released in the hydration. This is due to the hydrogen bonding in water, which means a lot of energy is needed to make it. Mixing the chemicals the other way. You Pour Water To Acid.
From h-o-m-e.org
The Right Way to Mix Acids and Water Always Add Acid to Water! You Pour Water To Acid When you add water to acid, the water will layer on top of the more densely concentrated acid or base. You should always add acid to water, otherwise you will end up with an extremely exothermic reaction that can even boil the water and cause violent bumping that can result in acid splashing all over the place. This is because. You Pour Water To Acid.
From mysafetylabels.com
Always Add Acid To Water ANSI Caution Sign Made In USA, SKU S20804 You Pour Water To Acid The extreme heat produced cause the solution to boil and project the upper layer out of the container. When you add water to acid, the water will layer on top of the more densely concentrated acid or base. Mixing the chemicals the other way can present a lab safety hazard. You should always add acid to water, otherwise you will. You Pour Water To Acid.
From www.youtube.com
Diluting of an acid acids, bases and salts class 10th chemistry You Pour Water To Acid Obtain the correct amounts of deionized (di) water in one beaker and acid in another. Mixing the chemicals the other way can present a lab safety hazard. When you mix concentrated sulfuric acid and water, you pour the acid into a larger volume of water. When you add water to acid, the water will layer on top of the more. You Pour Water To Acid.
From www.youtube.com
Animated Water to acid or acid to...... YouTube You Pour Water To Acid Mixing the chemicals the other way can present a lab safety hazard. When you mix acid with water, it's extremely important to add the acid to the water rather than the other way around. When you mix concentrated sulfuric acid and water, you pour the acid into a larger volume of water. The extreme heat produced cause the solution to. You Pour Water To Acid.
From slideplayer.com
Petroleum & Mining department Practical General chemistry ppt download You Pour Water To Acid This is because acid and water react in a vigorous exothermic reaction , releasing heat, sometimes boiling the liquid. This is due to the hydrogen bonding in water, which means a lot of energy is needed to make it. The extreme heat produced cause the solution to boil and project the upper layer out of the container. The reason this. You Pour Water To Acid.
From qest.org.au
Add acid to water Queensland Education Science Technicians You Pour Water To Acid When you add water to acid, the water will layer on top of the more densely concentrated acid or base. When you mix concentrated sulfuric acid and water, you pour the acid into a larger volume of water. The reason this occurs is due to the large amount of energy released in the hydration. This is due to the hydrogen. You Pour Water To Acid.
From www.accuformnmc.com
Always Add Acid To Water OSHA Caution Safety Sign MCHL702 You Pour Water To Acid When you mix concentrated sulfuric acid and water, you pour the acid into a larger volume of water. When you add water to acid, the water will layer on top of the more densely concentrated acid or base. The extreme heat produced cause the solution to boil and project the upper layer out of the container. This is referred to. You Pour Water To Acid.
From www.healthline.com
Your Guide to Forever Chemicals in the Water Supply You Pour Water To Acid This is because acid and water react in a vigorous exothermic reaction , releasing heat, sometimes boiling the liquid. When you add water to acid, the water will layer on top of the more densely concentrated acid or base. You should always add acid to water, otherwise you will end up with an extremely exothermic reaction that can even boil. You Pour Water To Acid.
From sciencenotes.org
Add Acid to Water or Water to Acid? Safely Diluting Acids You Pour Water To Acid This is because acid and water react in a vigorous exothermic reaction , releasing heat, sometimes boiling the liquid. You should always add acid to water, otherwise you will end up with an extremely exothermic reaction that can even boil the water and cause violent bumping that can result in acid splashing all over the place. When you mix acid. You Pour Water To Acid.
From netsolwater.com
What are alkaline water and acidic water? Netsol Water You Pour Water To Acid This is due to the hydrogen bonding in water, which means a lot of energy is needed to make it. Mixing the chemicals the other way can present a lab safety hazard. When you mix acid with water, it's extremely important to add the acid to the water rather than the other way around. The extreme heat produced cause the. You Pour Water To Acid.
From www.sciencephoto.com
Sulphuric acid added to water Stock Image A500/0577 Science Photo You Pour Water To Acid Obtain the correct amounts of deionized (di) water in one beaker and acid in another. The reason this occurs is due to the large amount of energy released in the hydration. This is referred to as dissociative energy release. This is because acid and water react in a vigorous exothermic reaction , releasing heat, sometimes boiling the liquid. When you. You Pour Water To Acid.
From www.youtube.com
What happen if we add acid into water? YouTube You Pour Water To Acid Slowly pour all the acid into the water. When you mix concentrated sulfuric acid and water, you pour the acid into a larger volume of water. When you add water to acid, the water will layer on top of the more densely concentrated acid or base. When you mix acid with water, it's extremely important to add the acid to. You Pour Water To Acid.
From www.slideserve.com
PPT Safety Rules PowerPoint Presentation, free download ID2995408 You Pour Water To Acid Mixing the chemicals the other way can present a lab safety hazard. When acid is poured into water, it flows down the flask and mixes much better, so no boiling occurs. This is referred to as dissociative energy release. This is because acid and water react in a vigorous exothermic reaction , releasing heat, sometimes boiling the liquid. You should. You Pour Water To Acid.
From www.youtube.com
why is it that the acid should be added to water and not You Pour Water To Acid When acid is poured into water, it flows down the flask and mixes much better, so no boiling occurs. The reason this occurs is due to the large amount of energy released in the hydration. When you add water to acid, the water will layer on top of the more densely concentrated acid or base. Obtain the correct amounts of. You Pour Water To Acid.
From app.jove.com
Watera BronstedLowry Acid and Base; Autoionization of Water Concept You Pour Water To Acid You should always add acid to water, otherwise you will end up with an extremely exothermic reaction that can even boil the water and cause violent bumping that can result in acid splashing all over the place. This is referred to as dissociative energy release. When you mix concentrated sulfuric acid and water, you pour the acid into a larger. You Pour Water To Acid.
From uhighlsu.web.fc2.com
when mixing acid and water You Pour Water To Acid When you add water to acid, the water will layer on top of the more densely concentrated acid or base. Slowly pour all the acid into the water. When acid is poured into water, it flows down the flask and mixes much better, so no boiling occurs. When you mix acid with water, it's extremely important to add the acid. You Pour Water To Acid.
From healthandsafetysigns.co.nz
Always add acid to water caution sign Health and Safety Signs You Pour Water To Acid When you add water to acid, the water will layer on top of the more densely concentrated acid or base. This is because acid and water react in a vigorous exothermic reaction , releasing heat, sometimes boiling the liquid. When you mix concentrated sulfuric acid and water, you pour the acid into a larger volume of water. Obtain the correct. You Pour Water To Acid.
From byjus.com
The correct way of making a solution of acid in water is to You Pour Water To Acid When you mix acid with water, it's extremely important to add the acid to the water rather than the other way around. This is because acid and water react in a vigorous exothermic reaction , releasing heat, sometimes boiling the liquid. The extreme heat produced cause the solution to boil and project the upper layer out of the container. Mixing. You Pour Water To Acid.
From www.slideserve.com
PPT Laboratory Safety Rules PowerPoint Presentation, free download You Pour Water To Acid When you add water to acid, the water will layer on top of the more densely concentrated acid or base. This is referred to as dissociative energy release. This is due to the hydrogen bonding in water, which means a lot of energy is needed to make it. When you mix acid with water, it's extremely important to add the. You Pour Water To Acid.
From www.slideserve.com
PPT Properties of Matter PowerPoint Presentation, free download ID You Pour Water To Acid Mixing the chemicals the other way can present a lab safety hazard. This is because acid and water react in a vigorous exothermic reaction , releasing heat, sometimes boiling the liquid. When you mix acid with water, it's extremely important to add the acid to the water rather than the other way around. Obtain the correct amounts of deionized (di). You Pour Water To Acid.
From www.hazard-signs.nz
Caution Always Add Water To Acid Sign Caution Signs HAZARD SIGNS NZ You Pour Water To Acid When you mix concentrated sulfuric acid and water, you pour the acid into a larger volume of water. When you mix acid with water, it's extremely important to add the acid to the water rather than the other way around. Obtain the correct amounts of deionized (di) water in one beaker and acid in another. This is referred to as. You Pour Water To Acid.
From www.sciencephoto.com
Adding Acid to Water Stock Image C030/8081 Science Photo Library You Pour Water To Acid This is because acid and water react in a vigorous exothermic reaction , releasing heat, sometimes boiling the liquid. The reason this occurs is due to the large amount of energy released in the hydration. This is referred to as dissociative energy release. When you add water to acid, the water will layer on top of the more densely concentrated. You Pour Water To Acid.