Ideal Gas Behavior Temperature And Pressure . Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the. Chemists sometimes make comparisons against a standard temperature and pressure (stp) for reporting properties of gases: Use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given volume. Use avogadro’s number to convert between. An ideal gas is one that follows the gas laws at all conditions of temperature and pressure. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states.
from www.youtube.com
Use avogadro’s number to convert between. An ideal gas is one that follows the gas laws at all conditions of temperature and pressure. Chemists sometimes make comparisons against a standard temperature and pressure (stp) for reporting properties of gases: The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the. Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. Use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given volume. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states.
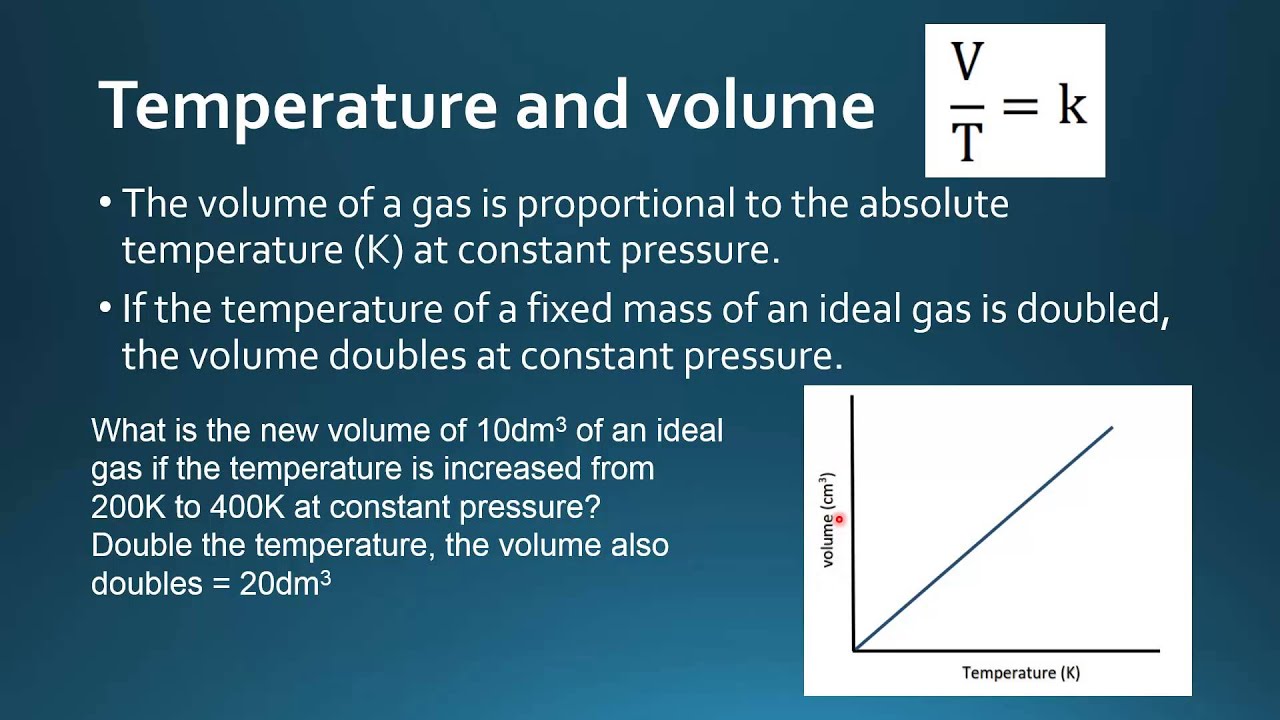
1.4.6 Solve problems involving temperature, pressure and volume for an ideal gas. YouTube
Ideal Gas Behavior Temperature And Pressure Chemists sometimes make comparisons against a standard temperature and pressure (stp) for reporting properties of gases: Use avogadro’s number to convert between. Use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given volume. Chemists sometimes make comparisons against a standard temperature and pressure (stp) for reporting properties of gases: Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. An ideal gas is one that follows the gas laws at all conditions of temperature and pressure. The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states.
From www.youtube.com
S1.5.1 and S1.5.2 Ideal gases and deviation from ideal gas behaviour YouTube Ideal Gas Behavior Temperature And Pressure An ideal gas is one that follows the gas laws at all conditions of temperature and pressure. Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. Chemists sometimes make comparisons against a standard temperature and pressure (stp) for reporting properties of gases: The ideal gas law describes the behavior of an. Ideal Gas Behavior Temperature And Pressure.
From www.dreamstime.com
Ideal Gas Law. Boyles Law Pressure Volume Relationship in Gases Stock Vector Illustration of Ideal Gas Behavior Temperature And Pressure Use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given volume. An ideal gas is one that follows the gas laws at all conditions of temperature and pressure. Chemists sometimes make comparisons against a standard temperature and pressure (stp) for reporting properties of gases: Ideal gas, a. Ideal Gas Behavior Temperature And Pressure.
From www.slideserve.com
PPT Chapter 14 The Behavior of Gases PowerPoint Presentation, free download ID9562086 Ideal Gas Behavior Temperature And Pressure Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. An ideal gas is one that follows the gas laws at all conditions of temperature and pressure. Use avogadro’s number to convert between. Chemists sometimes make comparisons against a standard temperature and pressure (stp) for reporting properties of gases: Use the ideal. Ideal Gas Behavior Temperature And Pressure.
From www.slideserve.com
PPT Gas Law and Gas Behavior PowerPoint Presentation, free download ID3183351 Ideal Gas Behavior Temperature And Pressure Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. An ideal gas is one that follows the gas laws at all conditions of temperature and pressure. Chemists sometimes make comparisons against a standard temperature and pressure (stp) for reporting properties of gases: Use. Ideal Gas Behavior Temperature And Pressure.
From owlcation.com
The Theories and Behavior of Gas Owlcation Ideal Gas Behavior Temperature And Pressure Use avogadro’s number to convert between. An ideal gas is one that follows the gas laws at all conditions of temperature and pressure. Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. Use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or. Ideal Gas Behavior Temperature And Pressure.
From owlcation.com
The Theories and Behavior of Gas Owlcation Ideal Gas Behavior Temperature And Pressure Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. Use avogadro’s number to convert between. The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the. An ideal gas is one that. Ideal Gas Behavior Temperature And Pressure.
From www.doubtnut.com
both pressure and temperature are high Ideal Gas Behavior Temperature And Pressure Use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given volume. Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and. Ideal Gas Behavior Temperature And Pressure.
From alevelphysicsnotes.com
Mr Toogood Physics The Experimental gas laws Ideal Gas Behavior Temperature And Pressure Chemists sometimes make comparisons against a standard temperature and pressure (stp) for reporting properties of gases: Use avogadro’s number to convert between. Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. Use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles. Ideal Gas Behavior Temperature And Pressure.
From www.slideserve.com
PPT The Ideal Gas Law 01 PowerPoint Presentation, free download ID3194926 Ideal Gas Behavior Temperature And Pressure An ideal gas is one that follows the gas laws at all conditions of temperature and pressure. Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. Chemists sometimes make comparisons against a standard temperature and pressure (stp) for reporting properties of gases: Ideal gas, a gas that conforms, in physical behavior,. Ideal Gas Behavior Temperature And Pressure.
From www.slideserve.com
PPT Chapter 14 The Behavior of Gases PowerPoint Presentation, free download ID9562086 Ideal Gas Behavior Temperature And Pressure An ideal gas is one that follows the gas laws at all conditions of temperature and pressure. Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the. Ideal gas, a. Ideal Gas Behavior Temperature And Pressure.
From sciencenotes.org
Ideal Gas Law Formula and Examples Ideal Gas Behavior Temperature And Pressure An ideal gas is one that follows the gas laws at all conditions of temperature and pressure. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can. Ideal Gas Behavior Temperature And Pressure.
From sciencenotes.org
Real Gas vs Ideal Gas Ideal Gas Behavior Temperature And Pressure Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. Use avogadro’s number to convert between. The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the. Chemists sometimes make comparisons against a. Ideal Gas Behavior Temperature And Pressure.
From www.youtube.com
Ideal Gas Pressure and Volume Dependence on Temperature YouTube Ideal Gas Behavior Temperature And Pressure Use avogadro’s number to convert between. Use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given volume. An ideal gas is one that follows the gas laws at all conditions of temperature and pressure. Chemists sometimes make comparisons against a standard temperature and pressure (stp) for reporting. Ideal Gas Behavior Temperature And Pressure.
From www.slideserve.com
PPT Ideal Gas Law PowerPoint Presentation, free download ID7067134 Ideal Gas Behavior Temperature And Pressure Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. Chemists sometimes make comparisons against a standard temperature and pressure (stp) for reporting properties of gases: Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states.. Ideal Gas Behavior Temperature And Pressure.
From www.bartleby.com
Ideal and Real Gases bartleby Ideal Gas Behavior Temperature And Pressure Chemists sometimes make comparisons against a standard temperature and pressure (stp) for reporting properties of gases: The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the. Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. Use the ideal gas. Ideal Gas Behavior Temperature And Pressure.
From saylordotorg.github.io
The Behavior of Real Gases Ideal Gas Behavior Temperature And Pressure Use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given volume. Use avogadro’s number to convert between. The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the. Chemists sometimes make comparisons against a standard temperature. Ideal Gas Behavior Temperature And Pressure.
From www.youtube.com
1.3 Deviation from ideal gas behaviour YouTube Ideal Gas Behavior Temperature And Pressure The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. Use avogadro’s number to convert between. Use the ideal gas law to. Ideal Gas Behavior Temperature And Pressure.
From 2012books.lardbucket.org
The Behavior of Real Gases Ideal Gas Behavior Temperature And Pressure Use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given volume. An ideal gas is one that follows the gas laws at all conditions of temperature and pressure. Use avogadro’s number to convert between. Chemists sometimes make comparisons against a standard temperature and pressure (stp) for reporting. Ideal Gas Behavior Temperature And Pressure.
From www.expii.com
Real vs. Ideal Gases — Comparison & Importance Expii Ideal Gas Behavior Temperature And Pressure Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. Use avogadro’s number to convert between. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. Chemists sometimes make comparisons against a standard temperature and pressure. Ideal Gas Behavior Temperature And Pressure.
From www.youtube.com
11.8 The Ideal Gas Law Pressure, Volume, Temperature, & Moles YouTube Ideal Gas Behavior Temperature And Pressure Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. An ideal gas is one that follows the gas laws at all conditions of temperature and pressure. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which. Ideal Gas Behavior Temperature And Pressure.
From www.britannica.com
perfect gas law chemistry and physics Britannica Ideal Gas Behavior Temperature And Pressure An ideal gas is one that follows the gas laws at all conditions of temperature and pressure. Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. Chemists sometimes make comparisons against a standard temperature and pressure (stp) for reporting properties of gases: Use the ideal gas law to calculate pressure change,. Ideal Gas Behavior Temperature And Pressure.
From www.slideserve.com
PPT REAL VS IDEAL GASES PowerPoint Presentation, free download ID1430890 Ideal Gas Behavior Temperature And Pressure Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. An ideal gas is one that follows the gas laws at all conditions of temperature and pressure. Use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given volume. The. Ideal Gas Behavior Temperature And Pressure.
From www.logiota.com
Deviation from Ideal Gas Behavior States of Matter Physical Chemistry Chemistry LOGiota Ideal Gas Behavior Temperature And Pressure Chemists sometimes make comparisons against a standard temperature and pressure (stp) for reporting properties of gases: The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the. Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. Use avogadro’s number to. Ideal Gas Behavior Temperature And Pressure.
From courses.lumenlearning.com
Relating Pressure, Volume, Amount, and Temperature The Ideal Gas Law CHEM 1305 Introductory Ideal Gas Behavior Temperature And Pressure An ideal gas is one that follows the gas laws at all conditions of temperature and pressure. Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. Chemists sometimes make comparisons against a standard temperature and pressure (stp) for reporting properties of gases: The ideal gas law describes the behavior of an. Ideal Gas Behavior Temperature And Pressure.
From www.ncbi.nlm.nih.gov
Ideal Gas Behavior StatPearls NCBI Bookshelf Ideal Gas Behavior Temperature And Pressure Use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given volume. Chemists sometimes make comparisons against a standard temperature and pressure (stp) for reporting properties of gases: The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively. Ideal Gas Behavior Temperature And Pressure.
From nigerianscholars.com
Phase Changes Temperature, Theory, and Gas Laws Ideal Gas Behavior Temperature And Pressure Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. An ideal gas is one that follows the gas laws at all conditions of temperature and pressure. Volume of a gas is directly proportional to the amount of gas at a constant temperature and. Ideal Gas Behavior Temperature And Pressure.
From www.slideserve.com
PPT Ideal Gas Equation PowerPoint Presentation, free download ID4187174 Ideal Gas Behavior Temperature And Pressure Use avogadro’s number to convert between. The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. Use the ideal gas law to. Ideal Gas Behavior Temperature And Pressure.
From classnotes.org.in
Real Gases Chemistry, Class 11, States of Matter Ideal Gas Behavior Temperature And Pressure An ideal gas is one that follows the gas laws at all conditions of temperature and pressure. Chemists sometimes make comparisons against a standard temperature and pressure (stp) for reporting properties of gases: Use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given volume. Use avogadro’s number. Ideal Gas Behavior Temperature And Pressure.
From www.grc.nasa.gov
Equation of State Ideal Gas Behavior Temperature And Pressure Use avogadro’s number to convert between. Chemists sometimes make comparisons against a standard temperature and pressure (stp) for reporting properties of gases: Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. The ideal gas law describes the behavior of an ideal gas, a. Ideal Gas Behavior Temperature And Pressure.
From www.youtube.com
1.4.6 Solve problems involving temperature, pressure and volume for an ideal gas. YouTube Ideal Gas Behavior Temperature And Pressure Use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given volume. Chemists sometimes make comparisons against a standard temperature and pressure (stp) for reporting properties of gases: Use avogadro’s number to convert between. Volume of a gas is directly proportional to the amount of gas at a. Ideal Gas Behavior Temperature And Pressure.
From chem.libretexts.org
1.1 The Perfect Gas Chemistry LibreTexts Ideal Gas Behavior Temperature And Pressure The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the. Chemists sometimes make comparisons against a standard temperature and pressure (stp) for reporting properties of gases: Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal. Ideal Gas Behavior Temperature And Pressure.
From owlcation.com
The Theories and Behavior of Gas Owlcation Ideal Gas Behavior Temperature And Pressure Chemists sometimes make comparisons against a standard temperature and pressure (stp) for reporting properties of gases: Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. Use avogadro’s number to convert between. The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively. Ideal Gas Behavior Temperature And Pressure.
From owlcation.com
The Theories and Behavior of Gas Owlcation Ideal Gas Behavior Temperature And Pressure Use avogadro’s number to convert between. Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. An ideal gas is one that follows the gas laws at all conditions of temperature and pressure. The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained. Ideal Gas Behavior Temperature And Pressure.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download ID6717792 Ideal Gas Behavior Temperature And Pressure Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. Chemists sometimes make comparisons against a standard temperature and pressure (stp) for reporting properties of gases: An ideal gas is one that follows the gas laws at all conditions of temperature and pressure. Use the ideal gas law to calculate pressure change,. Ideal Gas Behavior Temperature And Pressure.
From www.slideserve.com
PPT Behavior of Gases PowerPoint Presentation, free download ID3099616 Ideal Gas Behavior Temperature And Pressure Use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given volume. An ideal gas is one that follows the gas laws at all conditions of temperature and pressure. Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. Chemists. Ideal Gas Behavior Temperature And Pressure.