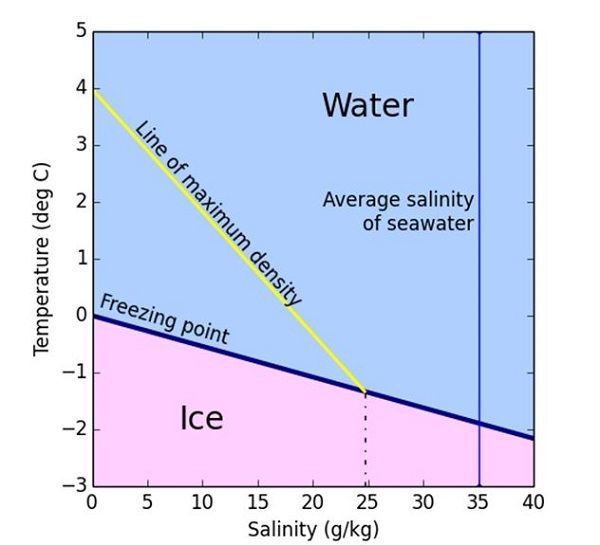
Salt Water Ice Melting Point . The more surface area salt can cover, the better the chances for melting ice. Salt lowers the water's freezing point via freezing point depression. When you mix salt onto that layer, it slowly lowers its melting point. When ice melts, water and ice coexist. At 0 °c, ice melts and freezes at the same rate, so you don't see ice melting at this temperature. The salt has to dissolve into its ions in order to work. Among other processes, the ions from the salt get in the way of water molecules aligning to crystallize into ice. At this temperature, your icy road generally has a thin layer of water on top of the ice, and the ice molecules and water molecules are interacting. Because salt particles make it harder for water particles to freeze back onto the ice, the ice that. Salt only helps if there is a little bit of liquid water available. When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it from freezing. This phenomenon is called freezing point depression.
from www.metoffice.gov.uk
This phenomenon is called freezing point depression. Among other processes, the ions from the salt get in the way of water molecules aligning to crystallize into ice. At this temperature, your icy road generally has a thin layer of water on top of the ice, and the ice molecules and water molecules are interacting. Because salt particles make it harder for water particles to freeze back onto the ice, the ice that. At 0 °c, ice melts and freezes at the same rate, so you don't see ice melting at this temperature. Salt only helps if there is a little bit of liquid water available. When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it from freezing. The more surface area salt can cover, the better the chances for melting ice. When you mix salt onto that layer, it slowly lowers its melting point. The salt has to dissolve into its ions in order to work.
Sea ice an overview Met Office
Salt Water Ice Melting Point At 0 °c, ice melts and freezes at the same rate, so you don't see ice melting at this temperature. This phenomenon is called freezing point depression. When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it from freezing. At 0 °c, ice melts and freezes at the same rate, so you don't see ice melting at this temperature. When ice melts, water and ice coexist. Salt only helps if there is a little bit of liquid water available. The more surface area salt can cover, the better the chances for melting ice. At this temperature, your icy road generally has a thin layer of water on top of the ice, and the ice molecules and water molecules are interacting. Salt lowers the water's freezing point via freezing point depression. Among other processes, the ions from the salt get in the way of water molecules aligning to crystallize into ice. The salt has to dissolve into its ions in order to work. When you mix salt onto that layer, it slowly lowers its melting point. Because salt particles make it harder for water particles to freeze back onto the ice, the ice that.
From studylib.net
Freezing Point of Salt Water .docx Salt Water Ice Melting Point At this temperature, your icy road generally has a thin layer of water on top of the ice, and the ice molecules and water molecules are interacting. Because salt particles make it harder for water particles to freeze back onto the ice, the ice that. This phenomenon is called freezing point depression. When rock salt is mixed in the water,. Salt Water Ice Melting Point.
From punchlistzero.com
Specific Heat of Ice In Various Units, vs. Water, Ice's Thermal Properties Salt Water Ice Melting Point The salt has to dissolve into its ions in order to work. When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it from freezing. The more surface area salt can cover, the better the chances for melting ice. Salt only helps if there is a little bit of liquid water available. When. Salt Water Ice Melting Point.
From www.scientificamerican.com
Salt Doesn't Melt IceHere's How It Makes Winter Streets Safer Scientific American Salt Water Ice Melting Point At this temperature, your icy road generally has a thin layer of water on top of the ice, and the ice molecules and water molecules are interacting. This phenomenon is called freezing point depression. The salt has to dissolve into its ions in order to work. When rock salt is mixed in the water, it lowers the freezing point of. Salt Water Ice Melting Point.
From 88guru.com
Melting Point of Ice and Boiling Point of Water 88Guru Salt Water Ice Melting Point The more surface area salt can cover, the better the chances for melting ice. Salt lowers the water's freezing point via freezing point depression. When you mix salt onto that layer, it slowly lowers its melting point. When ice melts, water and ice coexist. At 0 °c, ice melts and freezes at the same rate, so you don't see ice. Salt Water Ice Melting Point.
From www.themunicipal.com
Minimizing the impact of ice The Municipal Salt Water Ice Melting Point The more surface area salt can cover, the better the chances for melting ice. When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it from freezing. When you mix salt onto that layer, it slowly lowers its melting point. At 0 °c, ice melts and freezes at the same rate, so you. Salt Water Ice Melting Point.
From www.slideserve.com
PPT Physical and Chemical Properties 9/16/2014 PowerPoint Presentation ID5849998 Salt Water Ice Melting Point Salt lowers the water's freezing point via freezing point depression. When ice melts, water and ice coexist. This phenomenon is called freezing point depression. When you mix salt onto that layer, it slowly lowers its melting point. The more surface area salt can cover, the better the chances for melting ice. Salt only helps if there is a little bit. Salt Water Ice Melting Point.
From www.slideserve.com
PPT Salt & Freezing Point PowerPoint Presentation, free download ID5353727 Salt Water Ice Melting Point Salt lowers the water's freezing point via freezing point depression. When you mix salt onto that layer, it slowly lowers its melting point. When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it from freezing. The more surface area salt can cover, the better the chances for melting ice. This phenomenon is. Salt Water Ice Melting Point.
From materialmcgheepearter.z21.web.core.windows.net
How Does Salt Affect Ice Salt Water Ice Melting Point The more surface area salt can cover, the better the chances for melting ice. Salt lowers the water's freezing point via freezing point depression. Because salt particles make it harder for water particles to freeze back onto the ice, the ice that. When you mix salt onto that layer, it slowly lowers its melting point. Salt only helps if there. Salt Water Ice Melting Point.
From studiousguy.com
10 Melting Point Examples in Everyday Life StudiousGuy Salt Water Ice Melting Point Salt only helps if there is a little bit of liquid water available. The salt has to dissolve into its ions in order to work. The more surface area salt can cover, the better the chances for melting ice. When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it from freezing. When. Salt Water Ice Melting Point.
From paksc.org
Does adding salt to water lower its freezing point? Salt Water Ice Melting Point When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it from freezing. The salt has to dissolve into its ions in order to work. This phenomenon is called freezing point depression. At 0 °c, ice melts and freezes at the same rate, so you don't see ice melting at this temperature. At. Salt Water Ice Melting Point.
From www.researchgate.net
Salt hydrates with melting points below 100 °C. Download Table Salt Water Ice Melting Point The salt has to dissolve into its ions in order to work. Salt lowers the water's freezing point via freezing point depression. When ice melts, water and ice coexist. When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it from freezing. The more surface area salt can cover, the better the chances. Salt Water Ice Melting Point.
From snowicesalt.com
Ice Melt Comparison Chart Snow & Ice Salt & Chemicals Unlimited, LLC, A RASEVIC COMPANY Salt Water Ice Melting Point When ice melts, water and ice coexist. Among other processes, the ions from the salt get in the way of water molecules aligning to crystallize into ice. Salt lowers the water's freezing point via freezing point depression. This phenomenon is called freezing point depression. The more surface area salt can cover, the better the chances for melting ice. At 0. Salt Water Ice Melting Point.
From www.slideserve.com
PPT Salt Changes the Freezing Point of Water PowerPoint Presentation ID6060261 Salt Water Ice Melting Point The more surface area salt can cover, the better the chances for melting ice. When you mix salt onto that layer, it slowly lowers its melting point. The salt has to dissolve into its ions in order to work. Among other processes, the ions from the salt get in the way of water molecules aligning to crystallize into ice. Salt. Salt Water Ice Melting Point.
From ksa.mytutorsource.com
Water Freezing Point Definition, Factors Affecting It & Supercooled Water! MTS Blog Salt Water Ice Melting Point When you mix salt onto that layer, it slowly lowers its melting point. The salt has to dissolve into its ions in order to work. Among other processes, the ions from the salt get in the way of water molecules aligning to crystallize into ice. At this temperature, your icy road generally has a thin layer of water on top. Salt Water Ice Melting Point.
From sealevel.nasa.gov
Melting Ocean Ice Affects Sea Level Unlike Ice Cubes in a Glass NASA Sea Level Change Portal Salt Water Ice Melting Point Because salt particles make it harder for water particles to freeze back onto the ice, the ice that. When you mix salt onto that layer, it slowly lowers its melting point. The more surface area salt can cover, the better the chances for melting ice. This phenomenon is called freezing point depression. When ice melts, water and ice coexist. The. Salt Water Ice Melting Point.
From www.metoffice.gov.uk
Sea ice in the climate system Met Office Salt Water Ice Melting Point Salt lowers the water's freezing point via freezing point depression. When you mix salt onto that layer, it slowly lowers its melting point. Salt only helps if there is a little bit of liquid water available. Among other processes, the ions from the salt get in the way of water molecules aligning to crystallize into ice. When rock salt is. Salt Water Ice Melting Point.
From ksa.mytutorsource.com
Water Freezing Point Definition, Factors Affecting It & Supercooled Water! Salt Water Ice Melting Point Salt lowers the water's freezing point via freezing point depression. The salt has to dissolve into its ions in order to work. At 0 °c, ice melts and freezes at the same rate, so you don't see ice melting at this temperature. When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it. Salt Water Ice Melting Point.
From general.chemistrysteps.com
Freezing Point Depression Chemistry Steps Salt Water Ice Melting Point At this temperature, your icy road generally has a thin layer of water on top of the ice, and the ice molecules and water molecules are interacting. Because salt particles make it harder for water particles to freeze back onto the ice, the ice that. At 0 °c, ice melts and freezes at the same rate, so you don't see. Salt Water Ice Melting Point.
From www.slideserve.com
PPT Salt Changes the Freezing Point of Water PowerPoint Presentation ID6060261 Salt Water Ice Melting Point Because salt particles make it harder for water particles to freeze back onto the ice, the ice that. Salt only helps if there is a little bit of liquid water available. Salt lowers the water's freezing point via freezing point depression. The more surface area salt can cover, the better the chances for melting ice. At this temperature, your icy. Salt Water Ice Melting Point.
From www.worksheetsplanet.com
What is Melting Point Definition of Melting Point Salt Water Ice Melting Point When ice melts, water and ice coexist. Among other processes, the ions from the salt get in the way of water molecules aligning to crystallize into ice. This phenomenon is called freezing point depression. Salt only helps if there is a little bit of liquid water available. Salt lowers the water's freezing point via freezing point depression. The more surface. Salt Water Ice Melting Point.
From huntingwaterfalls.com
Why Does Salt Melt Ice? (Simple Science Explained) Salt Water Ice Melting Point This phenomenon is called freezing point depression. When you mix salt onto that layer, it slowly lowers its melting point. At 0 °c, ice melts and freezes at the same rate, so you don't see ice melting at this temperature. Because salt particles make it harder for water particles to freeze back onto the ice, the ice that. Salt only. Salt Water Ice Melting Point.
From www.researchgate.net
Freezing temperatures of salt brine mixtures Download Scientific Diagram Salt Water Ice Melting Point Salt lowers the water's freezing point via freezing point depression. Because salt particles make it harder for water particles to freeze back onto the ice, the ice that. At this temperature, your icy road generally has a thin layer of water on top of the ice, and the ice molecules and water molecules are interacting. This phenomenon is called freezing. Salt Water Ice Melting Point.
From www.slideserve.com
PPT Salt Changes the Freezing Point of Water PowerPoint Presentation ID6060261 Salt Water Ice Melting Point Salt only helps if there is a little bit of liquid water available. Among other processes, the ions from the salt get in the way of water molecules aligning to crystallize into ice. The more surface area salt can cover, the better the chances for melting ice. At 0 °c, ice melts and freezes at the same rate, so you. Salt Water Ice Melting Point.
From 88guru.com
Melting Point of Ice and Boiling Point of Water 88Guru Salt Water Ice Melting Point When you mix salt onto that layer, it slowly lowers its melting point. Salt lowers the water's freezing point via freezing point depression. Because salt particles make it harder for water particles to freeze back onto the ice, the ice that. Salt only helps if there is a little bit of liquid water available. This phenomenon is called freezing point. Salt Water Ice Melting Point.
From www.slideserve.com
PPT Melting Points and Mixed Melting Points PowerPoint Presentation, free download ID5757842 Salt Water Ice Melting Point At 0 °c, ice melts and freezes at the same rate, so you don't see ice melting at this temperature. Salt only helps if there is a little bit of liquid water available. The salt has to dissolve into its ions in order to work. When you mix salt onto that layer, it slowly lowers its melting point. Because salt. Salt Water Ice Melting Point.
From lessonlangdonslurp.z21.web.core.windows.net
What Happens When Salt And Ice Mix Salt Water Ice Melting Point Salt only helps if there is a little bit of liquid water available. At 0 °c, ice melts and freezes at the same rate, so you don't see ice melting at this temperature. Among other processes, the ions from the salt get in the way of water molecules aligning to crystallize into ice. When rock salt is mixed in the. Salt Water Ice Melting Point.
From www.youtube.com
Determination of melting point of ice and boiling point of water Chemistry Practical for Class Salt Water Ice Melting Point At 0 °c, ice melts and freezes at the same rate, so you don't see ice melting at this temperature. Salt lowers the water's freezing point via freezing point depression. Because salt particles make it harder for water particles to freeze back onto the ice, the ice that. When rock salt is mixed in the water, it lowers the freezing. Salt Water Ice Melting Point.
From saltsmart.org
How Does Salt Melt Snow and Ice? Salt Smart Collaborative Salt Water Ice Melting Point Salt only helps if there is a little bit of liquid water available. When ice melts, water and ice coexist. Among other processes, the ions from the salt get in the way of water molecules aligning to crystallize into ice. This phenomenon is called freezing point depression. Because salt particles make it harder for water particles to freeze back onto. Salt Water Ice Melting Point.
From www.metoffice.gov.uk
Sea ice an overview Met Office Salt Water Ice Melting Point Among other processes, the ions from the salt get in the way of water molecules aligning to crystallize into ice. When you mix salt onto that layer, it slowly lowers its melting point. Salt only helps if there is a little bit of liquid water available. This phenomenon is called freezing point depression. The salt has to dissolve into its. Salt Water Ice Melting Point.
From ar.inspiredpencil.com
Melting Point Of Ice Salt Water Ice Melting Point Salt lowers the water's freezing point via freezing point depression. The salt has to dissolve into its ions in order to work. Salt only helps if there is a little bit of liquid water available. This phenomenon is called freezing point depression. When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it. Salt Water Ice Melting Point.
From www.pinnaxis.com
What Is The Melting Point Of Ice Compare Discount Salt Water Ice Melting Point Among other processes, the ions from the salt get in the way of water molecules aligning to crystallize into ice. When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it from freezing. The salt has to dissolve into its ions in order to work. This phenomenon is called freezing point depression. When. Salt Water Ice Melting Point.
From www.youtube.com
Salt, Sugar or Hot Water Which melts ice faster? YouTube Salt Water Ice Melting Point The more surface area salt can cover, the better the chances for melting ice. Among other processes, the ions from the salt get in the way of water molecules aligning to crystallize into ice. When ice melts, water and ice coexist. The salt has to dissolve into its ions in order to work. This phenomenon is called freezing point depression.. Salt Water Ice Melting Point.
From www.worldatlas.com
Why Does Salt Melt Ice? WorldAtlas Salt Water Ice Melting Point When ice melts, water and ice coexist. At this temperature, your icy road generally has a thin layer of water on top of the ice, and the ice molecules and water molecules are interacting. Among other processes, the ions from the salt get in the way of water molecules aligning to crystallize into ice. When you mix salt onto that. Salt Water Ice Melting Point.
From www.youtube.com
Science Sunday Melting ice in salt water YouTube Salt Water Ice Melting Point The salt has to dissolve into its ions in order to work. Among other processes, the ions from the salt get in the way of water molecules aligning to crystallize into ice. Salt lowers the water's freezing point via freezing point depression. When rock salt is mixed in the water, it lowers the freezing point of the solution, preventing it. Salt Water Ice Melting Point.
From huntingwaterfalls.com
Why Does Salt Make Ice Colder? THE ACTUAL REASON Salt Water Ice Melting Point Among other processes, the ions from the salt get in the way of water molecules aligning to crystallize into ice. When ice melts, water and ice coexist. Because salt particles make it harder for water particles to freeze back onto the ice, the ice that. Salt lowers the water's freezing point via freezing point depression. This phenomenon is called freezing. Salt Water Ice Melting Point.