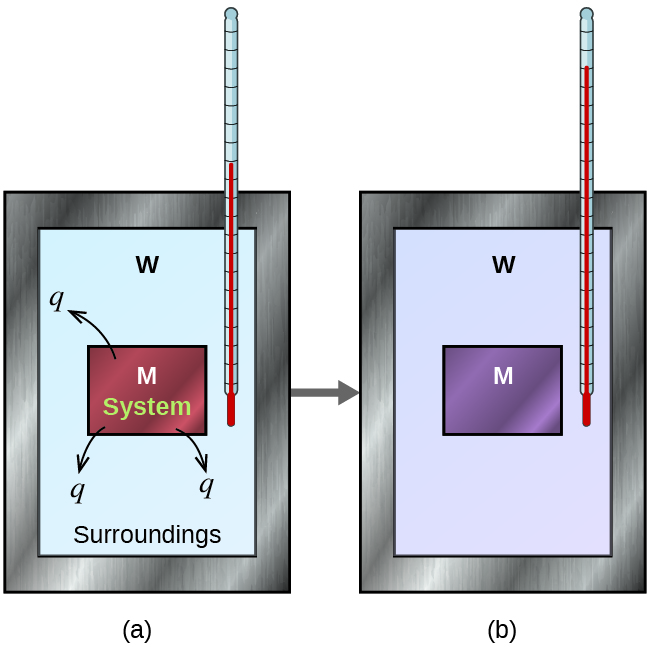
Calorimetry Type Of Reaction . Sections 11.5.1 and 11.5.2 will describe two common types of calorimeters designed for reactions taking place at either. A simple calorimeter can be made from a polystyrene drinking cup, a. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. It detects the heat change of a chemical reaction by directly measuring the temperature change it creates. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. One technique we can use to measure the amount of heat involved in a chemical or physical process is. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the measurement enthalpy changes in chemical reactions. For example, when an exothermic.
from wisc.pb.unizin.org
Apply the first law of thermodynamics to calorimetry. It detects the heat change of a chemical reaction by directly measuring the temperature change it creates. A simple calorimeter can be made from a polystyrene drinking cup, a. Sections 11.5.1 and 11.5.2 will describe two common types of calorimeters designed for reactions taking place at either. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Calorimetry is the measurement enthalpy changes in chemical reactions. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. One technique we can use to measure the amount of heat involved in a chemical or physical process is. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow
Calorimetry Type Of Reaction It detects the heat change of a chemical reaction by directly measuring the temperature change it creates. For example, when an exothermic reaction occurs in solution in a calorimeter, the. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. Sections 11.5.1 and 11.5.2 will describe two common types of calorimeters designed for reactions taking place at either. One technique we can use to measure the amount of heat involved in a chemical or physical process is. It detects the heat change of a chemical reaction by directly measuring the temperature change it creates. A simple calorimeter can be made from a polystyrene drinking cup, a. Calorimetry is the measurement enthalpy changes in chemical reactions. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Calorimetry Type Of Reaction Calorimetry is the measurement enthalpy changes in chemical reactions. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. It detects the heat change of a chemical reaction by directly measuring the temperature change it creates. A simple calorimeter can be made from a. Calorimetry Type Of Reaction.
From chem.libretexts.org
7.3 Heats of Reactions and Calorimetry Chemistry LibreTexts Calorimetry Type Of Reaction In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. For example, when an exothermic reaction occurs in solution in a calorimeter, the. For example, when an exothermic. Calorimetry is the measurement enthalpy changes in chemical reactions. It detects the heat change of a. Calorimetry Type Of Reaction.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 Calorimetry Type Of Reaction A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Calorimetry is the measurement enthalpy changes in chemical reactions. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. A calorimeter is a device used to measure the amount of heat. Calorimetry Type Of Reaction.
From chemistry.about.com
Coffee Cup and Bomb Calorimetry Calorimetry Type Of Reaction For example, when an exothermic reaction occurs in solution in a calorimeter, the. For example, when an exothermic. A simple calorimeter can be made from a polystyrene drinking cup, a. It detects the heat change of a chemical reaction by directly measuring the temperature change it creates. Calorimetry is the measurement enthalpy changes in chemical reactions. A calorimeter is a. Calorimetry Type Of Reaction.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Calorimetry Type Of Reaction A simple calorimeter can be made from a polystyrene drinking cup, a. For example, when an exothermic. It detects the heat change of a chemical reaction by directly measuring the temperature change it creates. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the measurement enthalpy changes in. Calorimetry Type Of Reaction.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Calorimetry Type Of Reaction In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. One technique we can use to measure the amount of heat involved in a chemical or physical process is. It detects the heat change of a chemical reaction by directly measuring the temperature change. Calorimetry Type Of Reaction.
From www.scribd.com
calorimetry Calorimetry Type Of Reaction Calorimetry is the measurement enthalpy changes in chemical reactions. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat. Calorimetry Type Of Reaction.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID1084959 Calorimetry Type Of Reaction Apply the first law of thermodynamics to calorimetry. A simple calorimeter can be made from a polystyrene drinking cup, a. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Sections 11.5.1 and 11.5.2 will describe two common types of calorimeters designed for reactions taking place at either. Calorimetry is the. Calorimetry Type Of Reaction.
From slideplayer.com
Calorimetry CP Unit 9 Chapter ppt download Calorimetry Type Of Reaction It detects the heat change of a chemical reaction by directly measuring the temperature change it creates. A simple calorimeter can be made from a polystyrene drinking cup, a. Sections 11.5.1 and 11.5.2 will describe two common types of calorimeters designed for reactions taking place at either. One technique we can use to measure the amount of heat involved in. Calorimetry Type Of Reaction.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Calorimetry Type Of Reaction A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It detects the heat change of a chemical reaction by directly measuring the temperature change it creates. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Compare heat flow from hot to cold objects in an ideal. Calorimetry Type Of Reaction.
From chemwiki.ucdavis.edu
Chapter 9.6 Calorimetry Chemwiki Calorimetry Type Of Reaction Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. For example, when an exothermic reaction occurs in solution in a calorimeter, the. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Apply the first law of thermodynamics to calorimetry. A calorimeter is a device. Calorimetry Type Of Reaction.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Calorimetry Type Of Reaction A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. A simple calorimeter can be made from a polystyrene drinking cup, a. For example, when an exothermic reaction occurs in solution in a calorimeter, the. A. Calorimetry Type Of Reaction.
From www.youtube.com
Types of calorimetry ?? Their advantages, Disadvantages & Use. YouTube Calorimetry Type Of Reaction In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. For example, when an exothermic. Sections 11.5.1 and 11.5.2 will describe two common types of calorimeters designed for reactions taking place at either. A calorimeter is a device used to measure the amount of. Calorimetry Type Of Reaction.
From www.numerade.com
SOLVED The following reactions were measured using a bomb calorimeter Calorimetry Type Of Reaction It detects the heat change of a chemical reaction by directly measuring the temperature change it creates. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Apply the first law of thermodynamics to calorimetry. One technique we can use to measure. Calorimetry Type Of Reaction.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Calorimetry Type Of Reaction A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the measurement enthalpy changes in chemical reactions. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. One technique we can use to measure the amount of heat involved in a chemical or physical. Calorimetry Type Of Reaction.
From www.studypool.com
SOLUTION Calorimetry Enthalpy of Reaction and Heat Capacity of a Calorimetry Type Of Reaction Apply the first law of thermodynamics to calorimetry. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Calorimetry is the measurement enthalpy changes in chemical reactions. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. In this article, you will learn about calorimetry and calorimeters, mainly. Calorimetry Type Of Reaction.
From wisc.pb.unizin.org
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow Calorimetry Type Of Reaction For example, when an exothermic. Sections 11.5.1 and 11.5.2 will describe two common types of calorimeters designed for reactions taking place at either. A simple calorimeter can be made from a polystyrene drinking cup, a. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. A calorimeter is a device used to measure the amount. Calorimetry Type Of Reaction.
From slideplayer.com
Calorimetry Chapter ppt download Calorimetry Type Of Reaction It detects the heat change of a chemical reaction by directly measuring the temperature change it creates. Sections 11.5.1 and 11.5.2 will describe two common types of calorimeters designed for reactions taking place at either. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. One technique we can use to. Calorimetry Type Of Reaction.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas Calorimetry Type Of Reaction A simple calorimeter can be made from a polystyrene drinking cup, a. It detects the heat change of a chemical reaction by directly measuring the temperature change it creates. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. In this article, you will learn about calorimetry and calorimeters, mainly used. Calorimetry Type Of Reaction.
From www.studypool.com
SOLUTION Calorimetry Enthalpy of Reaction and Heat Capacity of a Calorimetry Type Of Reaction For example, when an exothermic. It detects the heat change of a chemical reaction by directly measuring the temperature change it creates. Sections 11.5.1 and 11.5.2 will describe two common types of calorimeters designed for reactions taking place at either. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A. Calorimetry Type Of Reaction.
From chem.libretexts.org
11.5 Reaction Calorimetry Chemistry LibreTexts Calorimetry Type Of Reaction Sections 11.5.1 and 11.5.2 will describe two common types of calorimeters designed for reactions taking place at either. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It detects the heat change of a chemical reaction by directly measuring the temperature change it creates. One technique we can use to. Calorimetry Type Of Reaction.
From studylib.net
Calorimetry Calorimetry Type Of Reaction Sections 11.5.1 and 11.5.2 will describe two common types of calorimeters designed for reactions taking place at either. One technique we can use to measure the amount of heat involved in a chemical or physical process is. For example, when an exothermic. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and. Calorimetry Type Of Reaction.
From opentextbc.ca
5.2 Calorimetry Chemistry Calorimetry Type Of Reaction Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Sections 11.5.1 and 11.5.2 will describe two common types of calorimeters designed for reactions taking place at either. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. A. Calorimetry Type Of Reaction.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimetry Type Of Reaction Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Calorimetry is the measurement enthalpy changes in chemical reactions. Sections 11.5.1 and 11.5.2 will describe two common types of calorimeters designed for reactions taking place at either. In this article, you will learn about calorimetry and calorimeters,. Calorimetry Type Of Reaction.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Calorimetry Type Of Reaction For example, when an exothermic. It detects the heat change of a chemical reaction by directly measuring the temperature change it creates. Calorimetry is the measurement enthalpy changes in chemical reactions. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. For example, when an exothermic reaction occurs in solution in a calorimeter, the. One. Calorimetry Type Of Reaction.
From courses.lumenlearning.com
Calorimetry Introductory Chemistry Lecture & Lab Calorimetry Type Of Reaction Calorimetry is the measurement enthalpy changes in chemical reactions. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Apply the first law of thermodynamics to calorimetry. It detects the heat change of a chemical reaction by directly measuring the temperature change it creates. Sections 11.5.1 and 11.5.2 will describe two common types of calorimeters designed for. Calorimetry Type Of Reaction.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimetry Type Of Reaction A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. It detects the heat change of a chemical reaction by directly measuring the temperature change it creates. Calorimetry is the measurement enthalpy changes in chemical reactions.. Calorimetry Type Of Reaction.
From www.slideserve.com
PPT Energy changes in chemical reactions PowerPoint Presentation Calorimetry Type Of Reaction Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. One technique we can use to measure the amount of heat involved in a chemical or physical process is. For example, when an exothermic reaction occurs in solution in a calorimeter, the. A calorimeter is a device used to measure the amount of heat involved. Calorimetry Type Of Reaction.
From chem.libretexts.org
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts Calorimetry Type Of Reaction A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Sections 11.5.1 and 11.5.2 will describe two common types of calorimeters designed for reactions taking place at either. Apply the first law of thermodynamics to calorimetry. It detects the heat change of a chemical reaction by directly measuring the temperature change. Calorimetry Type Of Reaction.
From themasterchemistry.com
Calorimeter Types, Principle, Working, Uses Calorimetry Type Of Reaction It detects the heat change of a chemical reaction by directly measuring the temperature change it creates. Sections 11.5.1 and 11.5.2 will describe two common types of calorimeters designed for reactions taking place at either. One technique we can use to measure the amount of heat involved in a chemical or physical process is. For example, when an exothermic reaction. Calorimetry Type Of Reaction.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube Calorimetry Type Of Reaction One technique we can use to measure the amount of heat involved in a chemical or physical process is. A simple calorimeter can be made from a polystyrene drinking cup, a. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Apply the first law of thermodynamics to calorimetry. Calorimetry is the measurement enthalpy changes. Calorimetry Type Of Reaction.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Calorimetry Type Of Reaction Apply the first law of thermodynamics to calorimetry. One technique we can use to measure the amount of heat involved in a chemical or physical process is. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. For example, when an exothermic reaction occurs. Calorimetry Type Of Reaction.
From wisc.pb.unizin.org
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow Calorimetry Type Of Reaction Apply the first law of thermodynamics to calorimetry. One technique we can use to measure the amount of heat involved in a chemical or physical process is. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A simple calorimeter can be made from a polystyrene drinking cup, a. Sections 11.5.1. Calorimetry Type Of Reaction.
From www.chemistrystudent.com
Calorimetry (ALevel) ChemistryStudent Calorimetry Type Of Reaction For example, when an exothermic. A simple calorimeter can be made from a polystyrene drinking cup, a. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It detects the heat change of a chemical reaction by directly measuring the temperature change it creates. Apply the first law of thermodynamics to. Calorimetry Type Of Reaction.
From printablezonemarrow.z13.web.core.windows.net
How To Calculate Heat Of Reaction Calorimetry Calorimetry Type Of Reaction A simple calorimeter can be made from a polystyrene drinking cup, a. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Sections 11.5.1 and 11.5.2 will describe two common types of. Calorimetry Type Of Reaction.