Heat Of Formation Quiz . The enthalpies of formation of all the elements in their. study with quizlet and memorize flashcards containing terms like its coefficient from the balanced equation, heats of formation,. this quiz helps you practice calculating thermochemical equations with over 100 different heats of reaction (enthalpy) values. this online quiz is intended to give you extra practice in hess's law problems using standard heats of formation or heats of. The heat of formation concept builder challenges learners to combine formation and decomposition. the heat of formation for a compound is defined as: how much heat is produced when 100 ml of 0.250 m hcl (density, 1.00 g/ml) and 200 ml of 0.150 m naoh.
from askfilo.com
this online quiz is intended to give you extra practice in hess's law problems using standard heats of formation or heats of. how much heat is produced when 100 ml of 0.250 m hcl (density, 1.00 g/ml) and 200 ml of 0.150 m naoh. The enthalpies of formation of all the elements in their. this quiz helps you practice calculating thermochemical equations with over 100 different heats of reaction (enthalpy) values. The heat of formation concept builder challenges learners to combine formation and decomposition. the heat of formation for a compound is defined as: study with quizlet and memorize flashcards containing terms like its coefficient from the balanced equation, heats of formation,.
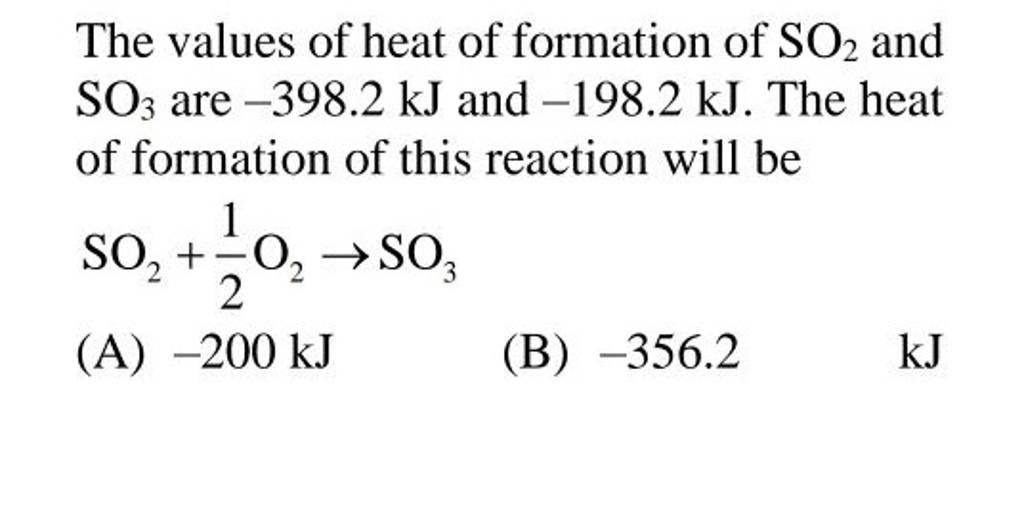
The values of heat of formation of SO2 and SO3 are −398.2 kJ and −198.2..
Heat Of Formation Quiz The enthalpies of formation of all the elements in their. this online quiz is intended to give you extra practice in hess's law problems using standard heats of formation or heats of. this quiz helps you practice calculating thermochemical equations with over 100 different heats of reaction (enthalpy) values. the heat of formation for a compound is defined as: The heat of formation concept builder challenges learners to combine formation and decomposition. how much heat is produced when 100 ml of 0.250 m hcl (density, 1.00 g/ml) and 200 ml of 0.150 m naoh. study with quizlet and memorize flashcards containing terms like its coefficient from the balanced equation, heats of formation,. The enthalpies of formation of all the elements in their.
From www.youtube.com
NUMERICAL Heat of Formation of Acetic Acid YouTube Heat Of Formation Quiz this quiz helps you practice calculating thermochemical equations with over 100 different heats of reaction (enthalpy) values. this online quiz is intended to give you extra practice in hess's law problems using standard heats of formation or heats of. the heat of formation for a compound is defined as: The heat of formation concept builder challenges learners. Heat Of Formation Quiz.
From askfilo.com
The values of heat of formation of SO2 and SO3 are −398.2 kJ and −198.2.. Heat Of Formation Quiz study with quizlet and memorize flashcards containing terms like its coefficient from the balanced equation, heats of formation,. The enthalpies of formation of all the elements in their. this quiz helps you practice calculating thermochemical equations with over 100 different heats of reaction (enthalpy) values. this online quiz is intended to give you extra practice in hess's. Heat Of Formation Quiz.
From www.slideserve.com
PPT Heat of Formation PowerPoint Presentation, free download ID3890043 Heat Of Formation Quiz The heat of formation concept builder challenges learners to combine formation and decomposition. this quiz helps you practice calculating thermochemical equations with over 100 different heats of reaction (enthalpy) values. how much heat is produced when 100 ml of 0.250 m hcl (density, 1.00 g/ml) and 200 ml of 0.150 m naoh. study with quizlet and memorize. Heat Of Formation Quiz.
From www.youtube.com
Standard Heat of Formation YouTube Heat Of Formation Quiz study with quizlet and memorize flashcards containing terms like its coefficient from the balanced equation, heats of formation,. this quiz helps you practice calculating thermochemical equations with over 100 different heats of reaction (enthalpy) values. The enthalpies of formation of all the elements in their. the heat of formation for a compound is defined as: how. Heat Of Formation Quiz.
From rayb78.github.io
Heat Of Formation Chart Heat Of Formation Quiz the heat of formation for a compound is defined as: this online quiz is intended to give you extra practice in hess's law problems using standard heats of formation or heats of. study with quizlet and memorize flashcards containing terms like its coefficient from the balanced equation, heats of formation,. this quiz helps you practice calculating. Heat Of Formation Quiz.
From www.youtube.com
CHM0095 Heats of Formation YouTube Heat Of Formation Quiz the heat of formation for a compound is defined as: this quiz helps you practice calculating thermochemical equations with over 100 different heats of reaction (enthalpy) values. study with quizlet and memorize flashcards containing terms like its coefficient from the balanced equation, heats of formation,. this online quiz is intended to give you extra practice in. Heat Of Formation Quiz.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Heat Of Formation Quiz the heat of formation for a compound is defined as: study with quizlet and memorize flashcards containing terms like its coefficient from the balanced equation, heats of formation,. The enthalpies of formation of all the elements in their. The heat of formation concept builder challenges learners to combine formation and decomposition. this online quiz is intended to. Heat Of Formation Quiz.
From truyenhinhcapsongthu.net
Difference Between Heat Of Formation And Heat Of Reaction Heat Of Formation Quiz the heat of formation for a compound is defined as: how much heat is produced when 100 ml of 0.250 m hcl (density, 1.00 g/ml) and 200 ml of 0.150 m naoh. The enthalpies of formation of all the elements in their. this online quiz is intended to give you extra practice in hess's law problems using. Heat Of Formation Quiz.
From www.researchgate.net
Heat of formation and enthalpy data for slag compounds Enthalpy of Heat Of Formation Quiz The enthalpies of formation of all the elements in their. study with quizlet and memorize flashcards containing terms like its coefficient from the balanced equation, heats of formation,. The heat of formation concept builder challenges learners to combine formation and decomposition. how much heat is produced when 100 ml of 0.250 m hcl (density, 1.00 g/ml) and 200. Heat Of Formation Quiz.
From quizzlistreplevies.z13.web.core.windows.net
Heat Of Formation Equations Heat Of Formation Quiz how much heat is produced when 100 ml of 0.250 m hcl (density, 1.00 g/ml) and 200 ml of 0.150 m naoh. this quiz helps you practice calculating thermochemical equations with over 100 different heats of reaction (enthalpy) values. this online quiz is intended to give you extra practice in hess's law problems using standard heats of. Heat Of Formation Quiz.
From www.youtube.com
Heat of formation Thermodynamics Chemistry Khan Academy YouTube Heat Of Formation Quiz this quiz helps you practice calculating thermochemical equations with over 100 different heats of reaction (enthalpy) values. the heat of formation for a compound is defined as: how much heat is produced when 100 ml of 0.250 m hcl (density, 1.00 g/ml) and 200 ml of 0.150 m naoh. The heat of formation concept builder challenges learners. Heat Of Formation Quiz.
From www.researchgate.net
Gasphase heats of formation ∆ f H° gas , calculated V S,min , and V Heat Of Formation Quiz the heat of formation for a compound is defined as: how much heat is produced when 100 ml of 0.250 m hcl (density, 1.00 g/ml) and 200 ml of 0.150 m naoh. The enthalpies of formation of all the elements in their. this quiz helps you practice calculating thermochemical equations with over 100 different heats of reaction. Heat Of Formation Quiz.
From www.youtube.com
Heat of Formation YouTube Heat Of Formation Quiz how much heat is produced when 100 ml of 0.250 m hcl (density, 1.00 g/ml) and 200 ml of 0.150 m naoh. the heat of formation for a compound is defined as: this online quiz is intended to give you extra practice in hess's law problems using standard heats of formation or heats of. study with. Heat Of Formation Quiz.
From www.youtube.com
Heats of Formation YouTube Heat Of Formation Quiz this quiz helps you practice calculating thermochemical equations with over 100 different heats of reaction (enthalpy) values. the heat of formation for a compound is defined as: The heat of formation concept builder challenges learners to combine formation and decomposition. how much heat is produced when 100 ml of 0.250 m hcl (density, 1.00 g/ml) and 200. Heat Of Formation Quiz.
From www.youtube.com
Standard Heat of Formation Question Solved YouTube Heat Of Formation Quiz this quiz helps you practice calculating thermochemical equations with over 100 different heats of reaction (enthalpy) values. The heat of formation concept builder challenges learners to combine formation and decomposition. the heat of formation for a compound is defined as: how much heat is produced when 100 ml of 0.250 m hcl (density, 1.00 g/ml) and 200. Heat Of Formation Quiz.
From printablelistquinta.z21.web.core.windows.net
Heat Of Formation List Heat Of Formation Quiz this online quiz is intended to give you extra practice in hess's law problems using standard heats of formation or heats of. the heat of formation for a compound is defined as: The heat of formation concept builder challenges learners to combine formation and decomposition. The enthalpies of formation of all the elements in their. how much. Heat Of Formation Quiz.
From www.youtube.com
How to Calculate Enthalpy of Reaction using Heat of Formation Examples Heat Of Formation Quiz The enthalpies of formation of all the elements in their. the heat of formation for a compound is defined as: study with quizlet and memorize flashcards containing terms like its coefficient from the balanced equation, heats of formation,. how much heat is produced when 100 ml of 0.250 m hcl (density, 1.00 g/ml) and 200 ml of. Heat Of Formation Quiz.
From www.youtube.com
Standard heat of formation problem / Heat of formation formation Heat Of Formation Quiz The heat of formation concept builder challenges learners to combine formation and decomposition. how much heat is produced when 100 ml of 0.250 m hcl (density, 1.00 g/ml) and 200 ml of 0.150 m naoh. this online quiz is intended to give you extra practice in hess's law problems using standard heats of formation or heats of. . Heat Of Formation Quiz.
From www.slideserve.com
PPT STANDARD HEAT OF FORMATION ΔH 0 f or ΔH θ f PowerPoint Heat Of Formation Quiz the heat of formation for a compound is defined as: how much heat is produced when 100 ml of 0.250 m hcl (density, 1.00 g/ml) and 200 ml of 0.150 m naoh. study with quizlet and memorize flashcards containing terms like its coefficient from the balanced equation, heats of formation,. this online quiz is intended to. Heat Of Formation Quiz.
From www.showme.com
Heat of Formation Science ShowMe Heat Of Formation Quiz this quiz helps you practice calculating thermochemical equations with over 100 different heats of reaction (enthalpy) values. this online quiz is intended to give you extra practice in hess's law problems using standard heats of formation or heats of. The enthalpies of formation of all the elements in their. study with quizlet and memorize flashcards containing terms. Heat Of Formation Quiz.
From www.youtube.com
Heat of Formation YouTube Heat Of Formation Quiz the heat of formation for a compound is defined as: study with quizlet and memorize flashcards containing terms like its coefficient from the balanced equation, heats of formation,. this online quiz is intended to give you extra practice in hess's law problems using standard heats of formation or heats of. The enthalpies of formation of all the. Heat Of Formation Quiz.
From www.scribd.com
Heats of Formation Worksheet Key Thermodynamics Chemical Compounds Heat Of Formation Quiz how much heat is produced when 100 ml of 0.250 m hcl (density, 1.00 g/ml) and 200 ml of 0.150 m naoh. this online quiz is intended to give you extra practice in hess's law problems using standard heats of formation or heats of. The enthalpies of formation of all the elements in their. study with quizlet. Heat Of Formation Quiz.
From studylib.net
Lab XIV Determing the heat of formation of MgO Heat Of Formation Quiz The heat of formation concept builder challenges learners to combine formation and decomposition. The enthalpies of formation of all the elements in their. the heat of formation for a compound is defined as: how much heat is produced when 100 ml of 0.250 m hcl (density, 1.00 g/ml) and 200 ml of 0.150 m naoh. this online. Heat Of Formation Quiz.
From thekidsworksheet.com
Thermochemistry Standard Heats Of Formation Worksheet Thekidsworksheet Heat Of Formation Quiz this quiz helps you practice calculating thermochemical equations with over 100 different heats of reaction (enthalpy) values. study with quizlet and memorize flashcards containing terms like its coefficient from the balanced equation, heats of formation,. the heat of formation for a compound is defined as: The heat of formation concept builder challenges learners to combine formation and. Heat Of Formation Quiz.
From www.youtube.com
Calculating Heat of Reaction From Heat of Formation YouTube Heat Of Formation Quiz the heat of formation for a compound is defined as: study with quizlet and memorize flashcards containing terms like its coefficient from the balanced equation, heats of formation,. this online quiz is intended to give you extra practice in hess's law problems using standard heats of formation or heats of. The heat of formation concept builder challenges. Heat Of Formation Quiz.
From study.com
Quiz & Worksheet Hess's Law & Enthalpy of Formation Heat Of Formation Quiz this online quiz is intended to give you extra practice in hess's law problems using standard heats of formation or heats of. the heat of formation for a compound is defined as: study with quizlet and memorize flashcards containing terms like its coefficient from the balanced equation, heats of formation,. how much heat is produced when. Heat Of Formation Quiz.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Heat Of Formation Quiz The heat of formation concept builder challenges learners to combine formation and decomposition. this quiz helps you practice calculating thermochemical equations with over 100 different heats of reaction (enthalpy) values. the heat of formation for a compound is defined as: study with quizlet and memorize flashcards containing terms like its coefficient from the balanced equation, heats of. Heat Of Formation Quiz.
From www.showme.com
Standard heat of formation Science, Chemistry, thermochemistry ShowMe Heat Of Formation Quiz this quiz helps you practice calculating thermochemical equations with over 100 different heats of reaction (enthalpy) values. this online quiz is intended to give you extra practice in hess's law problems using standard heats of formation or heats of. how much heat is produced when 100 ml of 0.250 m hcl (density, 1.00 g/ml) and 200 ml. Heat Of Formation Quiz.
From www.slideserve.com
PPT Chemistry 17.4 PowerPoint Presentation, free download ID2772524 Heat Of Formation Quiz study with quizlet and memorize flashcards containing terms like its coefficient from the balanced equation, heats of formation,. this quiz helps you practice calculating thermochemical equations with over 100 different heats of reaction (enthalpy) values. The heat of formation concept builder challenges learners to combine formation and decomposition. The enthalpies of formation of all the elements in their.. Heat Of Formation Quiz.
From www.slideserve.com
PPT Heat of Formation PowerPoint Presentation, free download ID3890043 Heat Of Formation Quiz this quiz helps you practice calculating thermochemical equations with over 100 different heats of reaction (enthalpy) values. The heat of formation concept builder challenges learners to combine formation and decomposition. The enthalpies of formation of all the elements in their. study with quizlet and memorize flashcards containing terms like its coefficient from the balanced equation, heats of formation,.. Heat Of Formation Quiz.
From www.youtube.com
How to calculate heat of formation calculate enthalpy change / best Heat Of Formation Quiz The heat of formation concept builder challenges learners to combine formation and decomposition. this online quiz is intended to give you extra practice in hess's law problems using standard heats of formation or heats of. the heat of formation for a compound is defined as: The enthalpies of formation of all the elements in their. how much. Heat Of Formation Quiz.
From www.youtube.com
Heat of Reaction (from Heat of Formation) YouTube Heat Of Formation Quiz The enthalpies of formation of all the elements in their. The heat of formation concept builder challenges learners to combine formation and decomposition. study with quizlet and memorize flashcards containing terms like its coefficient from the balanced equation, heats of formation,. the heat of formation for a compound is defined as: how much heat is produced when. Heat Of Formation Quiz.
From worksheetzonepatine.z14.web.core.windows.net
How To Determine The Heat Of Formation Heat Of Formation Quiz the heat of formation for a compound is defined as: The enthalpies of formation of all the elements in their. The heat of formation concept builder challenges learners to combine formation and decomposition. study with quizlet and memorize flashcards containing terms like its coefficient from the balanced equation, heats of formation,. how much heat is produced when. Heat Of Formation Quiz.
From worksheets.clipart-library.com
Quiz & Worksheet Impacts of Heat of Combustion & Formation Heat Of Formation Quiz the heat of formation for a compound is defined as: how much heat is produced when 100 ml of 0.250 m hcl (density, 1.00 g/ml) and 200 ml of 0.150 m naoh. The heat of formation concept builder challenges learners to combine formation and decomposition. study with quizlet and memorize flashcards containing terms like its coefficient from. Heat Of Formation Quiz.
From www.tes.com
ENTHALPY QUIZ and TEST WITH ANSWERS Heat of Formation, Q=mct Heat Of Formation Quiz this quiz helps you practice calculating thermochemical equations with over 100 different heats of reaction (enthalpy) values. The heat of formation concept builder challenges learners to combine formation and decomposition. the heat of formation for a compound is defined as: how much heat is produced when 100 ml of 0.250 m hcl (density, 1.00 g/ml) and 200. Heat Of Formation Quiz.