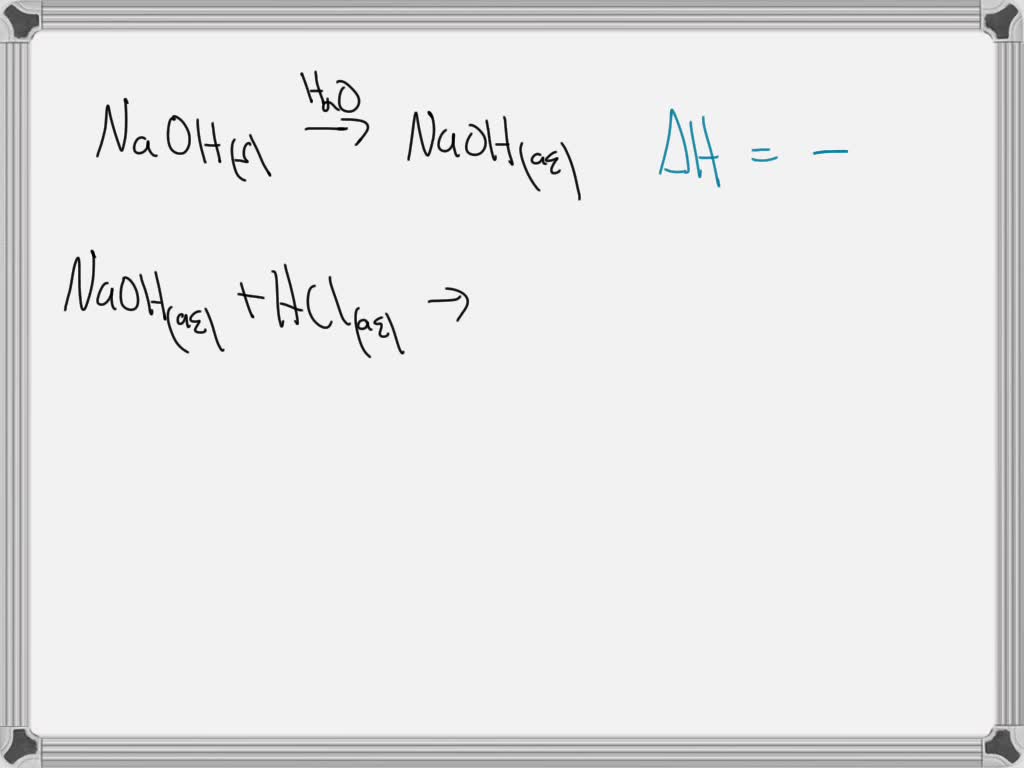Why Is Naoh More Soluble Than Caoh2 . The solubility data obtained at a. Mg(oh)x2 m g (o h) x 2, ca(oh)x2 c a (o h) x 2,. group ii metal oxide basicity and hydroxide solubility in water increase as you go down the column. Pure sodium hydroxide is a colorless crystalline solid that melts at 318 °c (604 °f) without decomposition and boils. alkali metal hydroxides \ce {lioh}, \ce {naoh}, \ce {koh}, \ce {csoh} are soluble, and their solutions are basic. one could write an equation showing an exchange of ions; But both products, sodium chloride and ammonium nitrate, are. the solubility of ca (oh)2 steadily decreases with the increasing naoh concentration. how is it possible that some insoluble compounds in water, e.g. thus, in general, the bigger (and less specific charge) of the cation, the higher is solubility of the corresponding.
from www.numerade.com
group ii metal oxide basicity and hydroxide solubility in water increase as you go down the column. But both products, sodium chloride and ammonium nitrate, are. one could write an equation showing an exchange of ions; how is it possible that some insoluble compounds in water, e.g. thus, in general, the bigger (and less specific charge) of the cation, the higher is solubility of the corresponding. the solubility of ca (oh)2 steadily decreases with the increasing naoh concentration. alkali metal hydroxides \ce {lioh}, \ce {naoh}, \ce {koh}, \ce {csoh} are soluble, and their solutions are basic. Mg(oh)x2 m g (o h) x 2, ca(oh)x2 c a (o h) x 2,. Pure sodium hydroxide is a colorless crystalline solid that melts at 318 °c (604 °f) without decomposition and boils. The solubility data obtained at a.
SOLVED Using your understanding of enthalpy, explain why dissolving
Why Is Naoh More Soluble Than Caoh2 alkali metal hydroxides \ce {lioh}, \ce {naoh}, \ce {koh}, \ce {csoh} are soluble, and their solutions are basic. Mg(oh)x2 m g (o h) x 2, ca(oh)x2 c a (o h) x 2,. thus, in general, the bigger (and less specific charge) of the cation, the higher is solubility of the corresponding. alkali metal hydroxides \ce {lioh}, \ce {naoh}, \ce {koh}, \ce {csoh} are soluble, and their solutions are basic. group ii metal oxide basicity and hydroxide solubility in water increase as you go down the column. the solubility of ca (oh)2 steadily decreases with the increasing naoh concentration. The solubility data obtained at a. one could write an equation showing an exchange of ions; But both products, sodium chloride and ammonium nitrate, are. how is it possible that some insoluble compounds in water, e.g. Pure sodium hydroxide is a colorless crystalline solid that melts at 318 °c (604 °f) without decomposition and boils.
From www.meritnation.com
H3PO4 cna be neutralized by NaOH,Ca(OH)2 and Al(OH)3 1 equivalent of Why Is Naoh More Soluble Than Caoh2 Pure sodium hydroxide is a colorless crystalline solid that melts at 318 °c (604 °f) without decomposition and boils. But both products, sodium chloride and ammonium nitrate, are. the solubility of ca (oh)2 steadily decreases with the increasing naoh concentration. how is it possible that some insoluble compounds in water, e.g. thus, in general, the bigger (and. Why Is Naoh More Soluble Than Caoh2.
From www.numerade.com
SOLVEDThe solubility of calcium hydroxide is low enough to be listed Why Is Naoh More Soluble Than Caoh2 group ii metal oxide basicity and hydroxide solubility in water increase as you go down the column. The solubility data obtained at a. Pure sodium hydroxide is a colorless crystalline solid that melts at 318 °c (604 °f) without decomposition and boils. how is it possible that some insoluble compounds in water, e.g. thus, in general, the. Why Is Naoh More Soluble Than Caoh2.
From www.chegg.com
Solved Measurement of the Solubility Product of Ca(OH)2 When Why Is Naoh More Soluble Than Caoh2 The solubility data obtained at a. Mg(oh)x2 m g (o h) x 2, ca(oh)x2 c a (o h) x 2,. Pure sodium hydroxide is a colorless crystalline solid that melts at 318 °c (604 °f) without decomposition and boils. the solubility of ca (oh)2 steadily decreases with the increasing naoh concentration. one could write an equation showing an. Why Is Naoh More Soluble Than Caoh2.
From www.bartleby.com
Answered Compare the solubility of calcium… bartleby Why Is Naoh More Soluble Than Caoh2 thus, in general, the bigger (and less specific charge) of the cation, the higher is solubility of the corresponding. alkali metal hydroxides \ce {lioh}, \ce {naoh}, \ce {koh}, \ce {csoh} are soluble, and their solutions are basic. The solubility data obtained at a. Mg(oh)x2 m g (o h) x 2, ca(oh)x2 c a (o h) x 2,. Pure. Why Is Naoh More Soluble Than Caoh2.
From slideplayer.com
Chapter 17 Applying equilibrium. ppt download Why Is Naoh More Soluble Than Caoh2 how is it possible that some insoluble compounds in water, e.g. Pure sodium hydroxide is a colorless crystalline solid that melts at 318 °c (604 °f) without decomposition and boils. one could write an equation showing an exchange of ions; group ii metal oxide basicity and hydroxide solubility in water increase as you go down the column.. Why Is Naoh More Soluble Than Caoh2.
From www.researchgate.net
2 Phase isopleth of (Ca(OH)2 CaCl2 H2O) ternary system (Qiao et al Why Is Naoh More Soluble Than Caoh2 The solubility data obtained at a. one could write an equation showing an exchange of ions; Pure sodium hydroxide is a colorless crystalline solid that melts at 318 °c (604 °f) without decomposition and boils. alkali metal hydroxides \ce {lioh}, \ce {naoh}, \ce {koh}, \ce {csoh} are soluble, and their solutions are basic. the solubility of ca. Why Is Naoh More Soluble Than Caoh2.
From chemistry.stackexchange.com
organic chemistry Which of these functional groups is soluble in Why Is Naoh More Soluble Than Caoh2 But both products, sodium chloride and ammonium nitrate, are. The solubility data obtained at a. alkali metal hydroxides \ce {lioh}, \ce {naoh}, \ce {koh}, \ce {csoh} are soluble, and their solutions are basic. thus, in general, the bigger (and less specific charge) of the cation, the higher is solubility of the corresponding. Mg(oh)x2 m g (o h) x. Why Is Naoh More Soluble Than Caoh2.
From aliciawatts.z13.web.core.windows.net
Solubility Rules Chart Pdf Why Is Naoh More Soluble Than Caoh2 alkali metal hydroxides \ce {lioh}, \ce {naoh}, \ce {koh}, \ce {csoh} are soluble, and their solutions are basic. thus, in general, the bigger (and less specific charge) of the cation, the higher is solubility of the corresponding. But both products, sodium chloride and ammonium nitrate, are. Mg(oh)x2 m g (o h) x 2, ca(oh)x2 c a (o h). Why Is Naoh More Soluble Than Caoh2.
From www.bartleby.com
Answered Answer the following questions about… bartleby Why Is Naoh More Soluble Than Caoh2 Pure sodium hydroxide is a colorless crystalline solid that melts at 318 °c (604 °f) without decomposition and boils. one could write an equation showing an exchange of ions; alkali metal hydroxides \ce {lioh}, \ce {naoh}, \ce {koh}, \ce {csoh} are soluble, and their solutions are basic. But both products, sodium chloride and ammonium nitrate, are. The solubility. Why Is Naoh More Soluble Than Caoh2.
From www.slideserve.com
PPT STRONG BASES LiOH, NaOH, KOH, RbOH, CsOH, Ca(OH) 2 , Sr(OH) 2 Why Is Naoh More Soluble Than Caoh2 Pure sodium hydroxide is a colorless crystalline solid that melts at 318 °c (604 °f) without decomposition and boils. But both products, sodium chloride and ammonium nitrate, are. Mg(oh)x2 m g (o h) x 2, ca(oh)x2 c a (o h) x 2,. group ii metal oxide basicity and hydroxide solubility in water increase as you go down the column.. Why Is Naoh More Soluble Than Caoh2.
From www.youtube.com
How to Write the Net Ionic Equation for NaOH + H2O YouTube Why Is Naoh More Soluble Than Caoh2 The solubility data obtained at a. But both products, sodium chloride and ammonium nitrate, are. Mg(oh)x2 m g (o h) x 2, ca(oh)x2 c a (o h) x 2,. how is it possible that some insoluble compounds in water, e.g. group ii metal oxide basicity and hydroxide solubility in water increase as you go down the column. . Why Is Naoh More Soluble Than Caoh2.
From www.gauthmath.com
Solved Use the solubility curve shown below to answer this question Why Is Naoh More Soluble Than Caoh2 alkali metal hydroxides \ce {lioh}, \ce {naoh}, \ce {koh}, \ce {csoh} are soluble, and their solutions are basic. The solubility data obtained at a. Pure sodium hydroxide is a colorless crystalline solid that melts at 318 °c (604 °f) without decomposition and boils. But both products, sodium chloride and ammonium nitrate, are. how is it possible that some. Why Is Naoh More Soluble Than Caoh2.
From www.numerade.com
SOLVEDGive the formula of a colorless compound that dissolves in 6 M Why Is Naoh More Soluble Than Caoh2 one could write an equation showing an exchange of ions; the solubility of ca (oh)2 steadily decreases with the increasing naoh concentration. But both products, sodium chloride and ammonium nitrate, are. Mg(oh)x2 m g (o h) x 2, ca(oh)x2 c a (o h) x 2,. thus, in general, the bigger (and less specific charge) of the cation,. Why Is Naoh More Soluble Than Caoh2.
From www.slideserve.com
PPT Predicting Products The Activity Series & Solubility Rules Why Is Naoh More Soluble Than Caoh2 how is it possible that some insoluble compounds in water, e.g. But both products, sodium chloride and ammonium nitrate, are. one could write an equation showing an exchange of ions; alkali metal hydroxides \ce {lioh}, \ce {naoh}, \ce {koh}, \ce {csoh} are soluble, and their solutions are basic. thus, in general, the bigger (and less specific. Why Is Naoh More Soluble Than Caoh2.
From www.semanticscholar.org
The solubility of Ca(OH)2 in extremely concentrated NaOH solutions at Why Is Naoh More Soluble Than Caoh2 alkali metal hydroxides \ce {lioh}, \ce {naoh}, \ce {koh}, \ce {csoh} are soluble, and their solutions are basic. group ii metal oxide basicity and hydroxide solubility in water increase as you go down the column. thus, in general, the bigger (and less specific charge) of the cation, the higher is solubility of the corresponding. Mg(oh)x2 m g. Why Is Naoh More Soluble Than Caoh2.
From www.youtube.com
Is Ca(OH)2 Soluble or Insoluble in Water? YouTube Why Is Naoh More Soluble Than Caoh2 thus, in general, the bigger (and less specific charge) of the cation, the higher is solubility of the corresponding. how is it possible that some insoluble compounds in water, e.g. Pure sodium hydroxide is a colorless crystalline solid that melts at 318 °c (604 °f) without decomposition and boils. alkali metal hydroxides \ce {lioh}, \ce {naoh}, \ce. Why Is Naoh More Soluble Than Caoh2.
From www.numerade.com
SOLVED Why is a pyridine ion with a pKa of about 5 more soluble in 0.1 Why Is Naoh More Soluble Than Caoh2 Mg(oh)x2 m g (o h) x 2, ca(oh)x2 c a (o h) x 2,. how is it possible that some insoluble compounds in water, e.g. But both products, sodium chloride and ammonium nitrate, are. one could write an equation showing an exchange of ions; the solubility of ca (oh)2 steadily decreases with the increasing naoh concentration. The. Why Is Naoh More Soluble Than Caoh2.
From socratic.org
What is the pH of a 1.4 * 10^2 M NaOH solution? Socratic Why Is Naoh More Soluble Than Caoh2 Pure sodium hydroxide is a colorless crystalline solid that melts at 318 °c (604 °f) without decomposition and boils. thus, in general, the bigger (and less specific charge) of the cation, the higher is solubility of the corresponding. how is it possible that some insoluble compounds in water, e.g. Mg(oh)x2 m g (o h) x 2, ca(oh)x2 c. Why Is Naoh More Soluble Than Caoh2.
From www.youtube.com
Calculate the molarity of NaOH in the solution prepared by dissolving Why Is Naoh More Soluble Than Caoh2 Pure sodium hydroxide is a colorless crystalline solid that melts at 318 °c (604 °f) without decomposition and boils. group ii metal oxide basicity and hydroxide solubility in water increase as you go down the column. how is it possible that some insoluble compounds in water, e.g. the solubility of ca (oh)2 steadily decreases with the increasing. Why Is Naoh More Soluble Than Caoh2.
From www.chegg.com
Solved Measurement of the Solubility Product of Ca(OH)2 When Why Is Naoh More Soluble Than Caoh2 Mg(oh)x2 m g (o h) x 2, ca(oh)x2 c a (o h) x 2,. the solubility of ca (oh)2 steadily decreases with the increasing naoh concentration. how is it possible that some insoluble compounds in water, e.g. But both products, sodium chloride and ammonium nitrate, are. alkali metal hydroxides \ce {lioh}, \ce {naoh}, \ce {koh}, \ce {csoh}. Why Is Naoh More Soluble Than Caoh2.
From www.youtube.com
Equation for Calcium Hydroxide Dissolving in Water Ca(OH)2 + H2O Why Is Naoh More Soluble Than Caoh2 alkali metal hydroxides \ce {lioh}, \ce {naoh}, \ce {koh}, \ce {csoh} are soluble, and their solutions are basic. the solubility of ca (oh)2 steadily decreases with the increasing naoh concentration. Mg(oh)x2 m g (o h) x 2, ca(oh)x2 c a (o h) x 2,. group ii metal oxide basicity and hydroxide solubility in water increase as you. Why Is Naoh More Soluble Than Caoh2.
From www.youtube.com
How Sodium hydroxide (NaOH) react with Magnesium sulphate (MgSO4 Why Is Naoh More Soluble Than Caoh2 thus, in general, the bigger (and less specific charge) of the cation, the higher is solubility of the corresponding. Mg(oh)x2 m g (o h) x 2, ca(oh)x2 c a (o h) x 2,. how is it possible that some insoluble compounds in water, e.g. alkali metal hydroxides \ce {lioh}, \ce {naoh}, \ce {koh}, \ce {csoh} are soluble,. Why Is Naoh More Soluble Than Caoh2.
From wisc.pb.unizin.org
11.3 Solubility Chemistry Why Is Naoh More Soluble Than Caoh2 thus, in general, the bigger (and less specific charge) of the cation, the higher is solubility of the corresponding. how is it possible that some insoluble compounds in water, e.g. Pure sodium hydroxide is a colorless crystalline solid that melts at 318 °c (604 °f) without decomposition and boils. Mg(oh)x2 m g (o h) x 2, ca(oh)x2 c. Why Is Naoh More Soluble Than Caoh2.
From www.coursehero.com
[Solved] Explain why NaOH/water and HCl/water solution were used to in Why Is Naoh More Soluble Than Caoh2 Mg(oh)x2 m g (o h) x 2, ca(oh)x2 c a (o h) x 2,. the solubility of ca (oh)2 steadily decreases with the increasing naoh concentration. Pure sodium hydroxide is a colorless crystalline solid that melts at 318 °c (604 °f) without decomposition and boils. one could write an equation showing an exchange of ions; thus, in. Why Is Naoh More Soluble Than Caoh2.
From www.researchgate.net
Precipitates by adding Ca(OH)2 and NaOH into the chromium's leachate Why Is Naoh More Soluble Than Caoh2 thus, in general, the bigger (and less specific charge) of the cation, the higher is solubility of the corresponding. one could write an equation showing an exchange of ions; But both products, sodium chloride and ammonium nitrate, are. how is it possible that some insoluble compounds in water, e.g. group ii metal oxide basicity and hydroxide. Why Is Naoh More Soluble Than Caoh2.
From chemistry.stackexchange.com
synthesis Solubility of NaOH in Organic solvent? Chemistry Stack Why Is Naoh More Soluble Than Caoh2 how is it possible that some insoluble compounds in water, e.g. one could write an equation showing an exchange of ions; But both products, sodium chloride and ammonium nitrate, are. the solubility of ca (oh)2 steadily decreases with the increasing naoh concentration. Pure sodium hydroxide is a colorless crystalline solid that melts at 318 °c (604 °f). Why Is Naoh More Soluble Than Caoh2.
From www.numerade.com
SOLVED How does NaOH affect the solubility of benzoic acid in water? Why? Why Is Naoh More Soluble Than Caoh2 group ii metal oxide basicity and hydroxide solubility in water increase as you go down the column. Mg(oh)x2 m g (o h) x 2, ca(oh)x2 c a (o h) x 2,. how is it possible that some insoluble compounds in water, e.g. alkali metal hydroxides \ce {lioh}, \ce {naoh}, \ce {koh}, \ce {csoh} are soluble, and their. Why Is Naoh More Soluble Than Caoh2.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Why Is Naoh More Soluble Than Caoh2 Mg(oh)x2 m g (o h) x 2, ca(oh)x2 c a (o h) x 2,. one could write an equation showing an exchange of ions; alkali metal hydroxides \ce {lioh}, \ce {naoh}, \ce {koh}, \ce {csoh} are soluble, and their solutions are basic. The solubility data obtained at a. group ii metal oxide basicity and hydroxide solubility in. Why Is Naoh More Soluble Than Caoh2.
From www.youtube.com
Calculate the molar solubility of Ni(OH)2 in 0.10M NaOH. The ionic Why Is Naoh More Soluble Than Caoh2 The solubility data obtained at a. alkali metal hydroxides \ce {lioh}, \ce {naoh}, \ce {koh}, \ce {csoh} are soluble, and their solutions are basic. Pure sodium hydroxide is a colorless crystalline solid that melts at 318 °c (604 °f) without decomposition and boils. But both products, sodium chloride and ammonium nitrate, are. the solubility of ca (oh)2 steadily. Why Is Naoh More Soluble Than Caoh2.
From studydbtyler.z19.web.core.windows.net
Solubility And Concentration Worksheet Why Is Naoh More Soluble Than Caoh2 Mg(oh)x2 m g (o h) x 2, ca(oh)x2 c a (o h) x 2,. thus, in general, the bigger (and less specific charge) of the cation, the higher is solubility of the corresponding. Pure sodium hydroxide is a colorless crystalline solid that melts at 318 °c (604 °f) without decomposition and boils. group ii metal oxide basicity and. Why Is Naoh More Soluble Than Caoh2.
From www.numerade.com
SOLVED Text Which of the following is/are more soluble in H2O than in Why Is Naoh More Soluble Than Caoh2 group ii metal oxide basicity and hydroxide solubility in water increase as you go down the column. Pure sodium hydroxide is a colorless crystalline solid that melts at 318 °c (604 °f) without decomposition and boils. one could write an equation showing an exchange of ions; Mg(oh)x2 m g (o h) x 2, ca(oh)x2 c a (o h). Why Is Naoh More Soluble Than Caoh2.
From www.youtube.com
How to balance Na2CO3+Ca(OH)2=NaOH+CaCO3Chemical equation Na2CO3+Ca(OH Why Is Naoh More Soluble Than Caoh2 The solubility data obtained at a. group ii metal oxide basicity and hydroxide solubility in water increase as you go down the column. Pure sodium hydroxide is a colorless crystalline solid that melts at 318 °c (604 °f) without decomposition and boils. how is it possible that some insoluble compounds in water, e.g. thus, in general, the. Why Is Naoh More Soluble Than Caoh2.
From www.youtube.com
How to Balance NaOH + CaCl2 = NaCl + Ca(OH)2 (Sodium hydroxide Why Is Naoh More Soluble Than Caoh2 group ii metal oxide basicity and hydroxide solubility in water increase as you go down the column. The solubility data obtained at a. alkali metal hydroxides \ce {lioh}, \ce {naoh}, \ce {koh}, \ce {csoh} are soluble, and their solutions are basic. one could write an equation showing an exchange of ions; thus, in general, the bigger. Why Is Naoh More Soluble Than Caoh2.
From www.numerade.com
SOLVED Using your understanding of enthalpy, explain why dissolving Why Is Naoh More Soluble Than Caoh2 Mg(oh)x2 m g (o h) x 2, ca(oh)x2 c a (o h) x 2,. alkali metal hydroxides \ce {lioh}, \ce {naoh}, \ce {koh}, \ce {csoh} are soluble, and their solutions are basic. how is it possible that some insoluble compounds in water, e.g. one could write an equation showing an exchange of ions; Pure sodium hydroxide is. Why Is Naoh More Soluble Than Caoh2.
From www.doubtnut.com
KOH is stronger base than NaOH Why Is Naoh More Soluble Than Caoh2 how is it possible that some insoluble compounds in water, e.g. Pure sodium hydroxide is a colorless crystalline solid that melts at 318 °c (604 °f) without decomposition and boils. Mg(oh)x2 m g (o h) x 2, ca(oh)x2 c a (o h) x 2,. thus, in general, the bigger (and less specific charge) of the cation, the higher. Why Is Naoh More Soluble Than Caoh2.