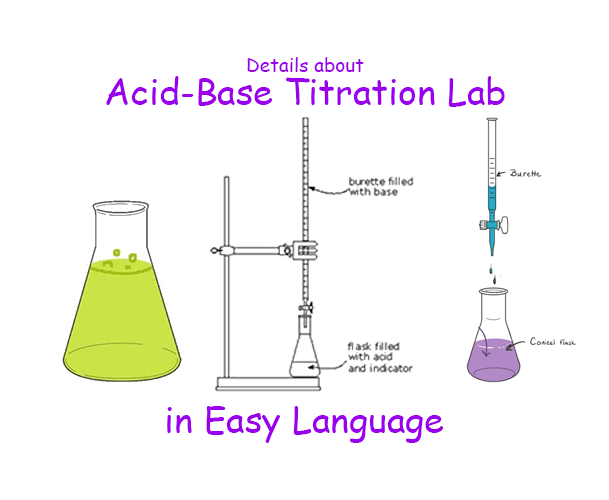
Lab Analysis Titration . Quantitative analysis of an unknown solution can be achieved using titration methods. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Conceptual problems the titration procedure is an application of. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as well as how titration is performed. How titrations work and what you need. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. Every titration uses the same players:
from mungfali.com
How titrations work and what you need. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Conceptual problems the titration procedure is an application of. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Every titration uses the same players: Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as well as how titration is performed. Quantitative analysis of an unknown solution can be achieved using titration methods.
Acid Base Titration Lab
Lab Analysis Titration Quantitative analysis of an unknown solution can be achieved using titration methods. Every titration uses the same players: Quantitative analysis of an unknown solution can be achieved using titration methods. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Conceptual problems the titration procedure is an application of. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as well as how titration is performed. How titrations work and what you need.
From www.scribd.com
LAB Report 3 Titration Chemistry Lab Analysis Titration Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. How titrations work and what you need. Quantitative analysis of an unknown solution can be achieved using titration methods. Conceptual problems the titration procedure is an application of. Titration is an analytical technique that allows the. Lab Analysis Titration.
From collegedunia.com
Volumetric Analysis Titration, Types, Principle & Procedure Lab Analysis Titration Conceptual problems the titration procedure is an application of. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Quantitative analysis of an unknown. Lab Analysis Titration.
From chem4three.blogspot.com
CHEMISTRY 11 Titration of Vinegar LAB Lab Analysis Titration How titrations work and what you need. Conceptual problems the titration procedure is an application of. Quantitative analysis of an unknown solution can be achieved using titration methods. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Titration is an analytical technique that allows the quantitative determination. Lab Analysis Titration.
From www.sliderbase.com
Titration Lab Analysis Titration How titrations work and what you need. Quantitative analysis of an unknown solution can be achieved using titration methods. Every titration uses the same players: Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Conceptual problems the titration procedure is an application of. Titration is an analytical. Lab Analysis Titration.
From childhealthpolicy.vumc.org
😱 Titration lab report discussion. Lab Report 9. 20221018 Lab Analysis Titration Quantitative analysis of an unknown solution can be achieved using titration methods. How titrations work and what you need. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Be able to determine the k a or k b from ph data associated with the titration. Lab Analysis Titration.
From www.birmingham.ac.uk
Chemistry titrations University of Birmingham Lab Analysis Titration Quantitative analysis of an unknown solution can be achieved using titration methods. How titrations work and what you need. Conceptual problems the titration procedure is an application of. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Every titration uses the same players: In this tutorial, you. Lab Analysis Titration.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Lab Analysis Titration Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as well as how titration is performed. Conceptual problems the titration procedure. Lab Analysis Titration.
From www.numerade.com
Titration for Acetic Acid in Vinegar Lab Report Exercise 1 Lab Analysis Titration Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. Conceptual problems the titration procedure is an application of. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as well as how. Lab Analysis Titration.
From about.dataclassroom.com
AcidBase Titration Lab — DataClassroom Lab Analysis Titration How titrations work and what you need. Conceptual problems the titration procedure is an application of. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make. Lab Analysis Titration.
From www.alamy.com
Analytical chemistry titration equipment. Laboratory glassware in a Lab Analysis Titration Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. How titrations work and what you need. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as well as how titration. Lab Analysis Titration.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS Lab Analysis Titration Quantitative analysis of an unknown solution can be achieved using titration methods. Conceptual problems the titration procedure is an application of. Every titration uses the same players: Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. How titrations work and what you need. In this tutorial, you. Lab Analysis Titration.
From mungfali.com
Titration Lab Diagram Lab Analysis Titration Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. How titrations work and what you need. In this tutorial, you will find. Lab Analysis Titration.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Lab Analysis Titration Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as well as how titration is performed. Quantitative analysis of an unknown. Lab Analysis Titration.
From ar.inspiredpencil.com
Titration Setup Diagram Lab Analysis Titration How titrations work and what you need. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Quantitative analysis of an unknown solution can be achieved using titration methods. Every titration uses the same players: Conceptual problems the titration procedure is an application of. Titration is an analytical. Lab Analysis Titration.
From bramblechemistry.weebly.com
4C6 Titration Lab Analysis Titration Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as well as how titration is performed. Conceptual problems the titration procedure. Lab Analysis Titration.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Lab Analysis Titration Every titration uses the same players: Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Quantitative analysis of an unknown solution can be. Lab Analysis Titration.
From www.scribd.com
titration ( chemistry Experiment report) Titration Analysis Lab Analysis Titration How titrations work and what you need. Conceptual problems the titration procedure is an application of. Quantitative analysis of an unknown solution can be achieved using titration methods. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Be able to determine the k a or. Lab Analysis Titration.
From www.youtube.com
Lab 8 Volumetric Analysis, An AcidBase Titration YouTube Lab Analysis Titration Every titration uses the same players: Conceptual problems the titration procedure is an application of. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. Quantitative analysis of an unknown solution can be achieved using titration methods. Titration is the slow addition of one solution of a. Lab Analysis Titration.
From www.chemicals.co.uk
What is Titration Used For in Real Life? The Chemistry Blog Lab Analysis Titration Conceptual problems the titration procedure is an application of. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Be able to determine. Lab Analysis Titration.
From www.vrogue.co
Titration Illustration vrogue.co Lab Analysis Titration Quantitative analysis of an unknown solution can be achieved using titration methods. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. In this. Lab Analysis Titration.
From pubs.rsc.org
A convenient chemical titration method to measure M( ii )/M( iii Lab Analysis Titration Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Quantitative analysis of an unknown solution can be achieved using titration methods. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. In this. Lab Analysis Titration.
From chem.libretexts.org
11 Titration of Vinegar (Experiment) Chemistry LibreTexts Lab Analysis Titration In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as well as how titration is performed. Conceptual problems the titration procedure is an application of. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition. Lab Analysis Titration.
From mungfali.com
Acid Base Titration Lab Lab Analysis Titration Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. Every titration uses the same players: Quantitative analysis of an unknown solution can. Lab Analysis Titration.
From springofchemistry.blogspot.com
Spring Of Chemistry Diagram for titration Lab Analysis Titration Every titration uses the same players: Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Conceptual problems the titration procedure is an application of. How titrations work and what you need. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in. Lab Analysis Titration.
From www.flinnsci.com
AcidBase Titrations—Classic Lab Kit for AP® Chemistry Flinn Scientific Lab Analysis Titration Quantitative analysis of an unknown solution can be achieved using titration methods. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as. Lab Analysis Titration.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Lab Analysis Titration Conceptual problems the titration procedure is an application of. Quantitative analysis of an unknown solution can be achieved using titration methods. Every titration uses the same players: How titrations work and what you need. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. Titration is the. Lab Analysis Titration.
From ctdvdimri.blogspot.com
Chemistry Honors Blog Lab 14 Titration Lab Lab Analysis Titration Quantitative analysis of an unknown solution can be achieved using titration methods. Every titration uses the same players: Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Be able to determine the k a or k b from ph data associated with the titration of. Lab Analysis Titration.
From analysisdoo.com
Titration Systems Archives Analysis Lab Analysis Titration Conceptual problems the titration procedure is an application of. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as well as how titration is performed. Quantitative analysis of an unknown solution can be achieved using titration methods. Titration is the slow addition of one solution. Lab Analysis Titration.
From chemistrylabs-2.blogspot.com
Titration Apparatus Diagram Chemistry Labs Lab Analysis Titration Quantitative analysis of an unknown solution can be achieved using titration methods. Conceptual problems the titration procedure is an application of. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a. Lab Analysis Titration.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Lab Analysis Titration Quantitative analysis of an unknown solution can be achieved using titration methods. Conceptual problems the titration procedure is an application of. How titrations work and what you need. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. In this tutorial, you will find information on titration,. Lab Analysis Titration.
From theedge.com.hk
Chemistry How To Titration The Edge Lab Analysis Titration Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. Every titration uses the same players: How titrations work and what you need.. Lab Analysis Titration.
From sciencephotogallery.com
Titration Experiment 8 by Lab Analysis Titration Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as well as how titration is performed. Quantitative analysis of an unknown. Lab Analysis Titration.
From betterlesson.com
Eleventh grade Lesson Titration Lab BetterLesson Lab Analysis Titration Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. How titrations work and what you need. Quantitative analysis of an unknown solution. Lab Analysis Titration.
From www.alamy.com
Titration, titrimetry or volumetric analysis. A burette and Erlenmeyer Lab Analysis Titration Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as well as how titration is performed. Quantitative analysis of an unknown. Lab Analysis Titration.
From www.microlit.com
An Advanced Guide to Titration Microlit Lab Analysis Titration In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as well as how titration is performed. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Conceptual problems the titration procedure is an. Lab Analysis Titration.