Ph Jump Titration . Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution. Titration curves graphically represent the change in ph as titrant is added. So what we need is to find an equation where the only. The equivalence point of a titration. See example 9.1 and check for understanding 9.1. This section describes what information these curves provide and how that information is used in chemistry. What are ph titration curves? Which indicator would be suitable for the titration of: In general, there are two requirements for a clearly discernible jump in the ph to occur in a polyprotic titration: See titration curves for acids and bases. Titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. The successive k a 's must differ by.
from studylib.net
Titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution. What are ph titration curves? In general, there are two requirements for a clearly discernible jump in the ph to occur in a polyprotic titration: Titration curves graphically represent the change in ph as titrant is added. So what we need is to find an equation where the only. The successive k a 's must differ by. Which indicator would be suitable for the titration of: The equivalence point of a titration. This section describes what information these curves provide and how that information is used in chemistry.
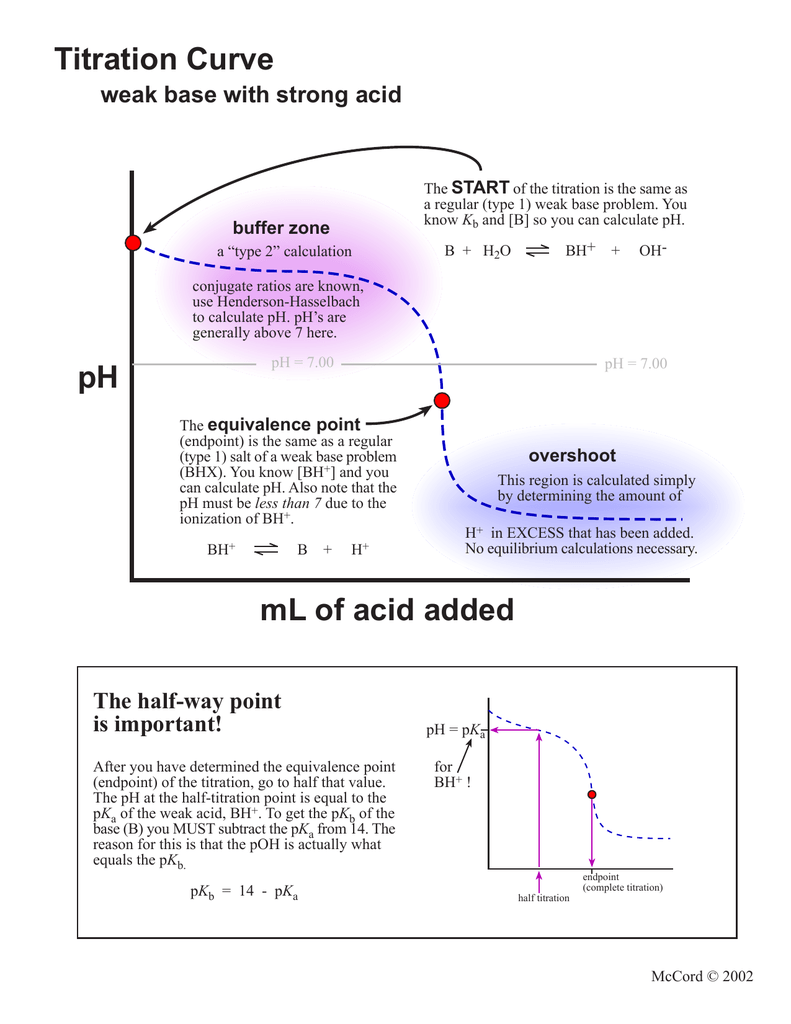
Titration Curve weak base with strong acid START
Ph Jump Titration Titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. This section describes what information these curves provide and how that information is used in chemistry. In general, there are two requirements for a clearly discernible jump in the ph to occur in a polyprotic titration: What are ph titration curves? Which indicator would be suitable for the titration of: The successive k a 's must differ by. Titration curves graphically represent the change in ph as titrant is added. See example 9.1 and check for understanding 9.1. Titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. See titration curves for acids and bases. The equivalence point of a titration. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution. So what we need is to find an equation where the only.
From www.savemyexams.com
pH Titration Curves CIE A Level Chemistry Revision Notes 2022 Ph Jump Titration The successive k a 's must differ by. What are ph titration curves? So what we need is to find an equation where the only. In general, there are two requirements for a clearly discernible jump in the ph to occur in a polyprotic titration: See example 9.1 and check for understanding 9.1. Titration is an analytical chemistry technique used. Ph Jump Titration.
From saylordotorg.github.io
AcidBase Titrations Ph Jump Titration Titration curves graphically represent the change in ph as titrant is added. In general, there are two requirements for a clearly discernible jump in the ph to occur in a polyprotic titration: Which indicator would be suitable for the titration of: See titration curves for acids and bases. What are ph titration curves? The equivalence point of a titration. See. Ph Jump Titration.
From www.researchgate.net
Potentiometric titration curve of lignosulfonates. ERC, Δ pH/ΔV; EP1 Ph Jump Titration See titration curves for acids and bases. The equivalence point of a titration. The successive k a 's must differ by. What are ph titration curves? So what we need is to find an equation where the only. In general, there are two requirements for a clearly discernible jump in the ph to occur in a polyprotic titration: Titration is. Ph Jump Titration.
From scienceready.com.au
Titration pH Curves HSC Chemistry Science Ready Ph Jump Titration In general, there are two requirements for a clearly discernible jump in the ph to occur in a polyprotic titration: See example 9.1 and check for understanding 9.1. This section describes what information these curves provide and how that information is used in chemistry. Which indicator would be suitable for the titration of: What are ph titration curves? The successive. Ph Jump Titration.
From www.chegg.com
Solved 1. Figure 1 shows the titration curves of four Ph Jump Titration So what we need is to find an equation where the only. Titration curves graphically represent the change in ph as titrant is added. The successive k a 's must differ by. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution. Titration is an analytical chemistry technique used to. Ph Jump Titration.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Ph Jump Titration In general, there are two requirements for a clearly discernible jump in the ph to occur in a polyprotic titration: See example 9.1 and check for understanding 9.1. Which indicator would be suitable for the titration of: See titration curves for acids and bases. This section describes what information these curves provide and how that information is used in chemistry.. Ph Jump Titration.
From scienceready.com.au
Titration pH Curves HSC Chemistry Science Ready Ph Jump Titration Titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. See example 9.1 and check for understanding 9.1. See titration curves for acids and bases. In general, there are two requirements for a clearly discernible jump in the ph to occur in a polyprotic titration: Which indicator would be suitable for the titration. Ph Jump Titration.
From www.researchgate.net
pH titration curve against strong ion difference (SID). The dotted line Ph Jump Titration The equivalence point of a titration. See titration curves for acids and bases. Titration curves graphically represent the change in ph as titrant is added. Which indicator would be suitable for the titration of: This section describes what information these curves provide and how that information is used in chemistry. The successive k a 's must differ by. See example. Ph Jump Titration.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Ph Jump Titration In general, there are two requirements for a clearly discernible jump in the ph to occur in a polyprotic titration: So what we need is to find an equation where the only. See titration curves for acids and bases. Which indicator would be suitable for the titration of: The equivalence point of a titration. The successive k a 's must. Ph Jump Titration.
From www.rms-foundation.ch
Titrando (Titrationen + pH Messung) RMS Foundation Ph Jump Titration Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution. Titration curves graphically represent the change in ph as titrant is added. The equivalence point of a titration. Which indicator would be suitable for the titration of: In general, there are two requirements for a clearly discernible jump in the. Ph Jump Titration.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Ph Jump Titration What are ph titration curves? Titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution. In general, there are two requirements for a clearly discernible jump in the ph to occur in a. Ph Jump Titration.
From solvedlib.com
The following graph shows the pH curve for the titrat… SolvedLib Ph Jump Titration Titration curves graphically represent the change in ph as titrant is added. Which indicator would be suitable for the titration of: See example 9.1 and check for understanding 9.1. So what we need is to find an equation where the only. The equivalence point of a titration. Titration is an analytical chemistry technique used to find the concentration of an. Ph Jump Titration.
From mmerevise.co.uk
pH Curves Questions and Revision MME Ph Jump Titration Titration curves graphically represent the change in ph as titrant is added. See example 9.1 and check for understanding 9.1. What are ph titration curves? So what we need is to find an equation where the only. See titration curves for acids and bases. This section describes what information these curves provide and how that information is used in chemistry.. Ph Jump Titration.
From staff.buffalostate.edu
AcidBase Reactions Ph Jump Titration Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution. What are ph titration curves? The successive k a 's must differ by. See titration curves for acids and bases. The equivalence point of a titration. Titration is an analytical chemistry technique used to find the concentration of an unknown. Ph Jump Titration.
From www.researchgate.net
Potentiometric titration curve of lignosulfonates. ERC, Δ pH/ΔV; EP1 Ph Jump Titration See example 9.1 and check for understanding 9.1. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution. The successive k a 's must differ by. What are ph titration curves? Titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. So what. Ph Jump Titration.
From 88guru.com
Acid Base Titration What is a Titration Curve? 88guru Ph Jump Titration The equivalence point of a titration. See example 9.1 and check for understanding 9.1. The successive k a 's must differ by. Titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution. What. Ph Jump Titration.
From mmerevise.co.uk
Acids and Bases Questions and Revision MME Ph Jump Titration What are ph titration curves? Titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. The successive k a 's must differ by. In general, there are two requirements for a clearly discernible jump in the ph to occur in a polyprotic titration: See example 9.1 and check for understanding 9.1. The equivalence. Ph Jump Titration.
From www.slideshare.net
pH Understanding titration curve Ph Jump Titration See example 9.1 and check for understanding 9.1. In general, there are two requirements for a clearly discernible jump in the ph to occur in a polyprotic titration: So what we need is to find an equation where the only. What are ph titration curves? The successive k a 's must differ by. This section describes what information these curves. Ph Jump Titration.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Ph Jump Titration This section describes what information these curves provide and how that information is used in chemistry. In general, there are two requirements for a clearly discernible jump in the ph to occur in a polyprotic titration: What are ph titration curves? See titration curves for acids and bases. Which indicator would be suitable for the titration of: Titration is an. Ph Jump Titration.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation Ph Jump Titration Which indicator would be suitable for the titration of: Titration curves graphically represent the change in ph as titrant is added. The successive k a 's must differ by. This section describes what information these curves provide and how that information is used in chemistry. Titration is an analytical chemistry technique used to find the concentration of an unknown acid. Ph Jump Titration.
From saylordotorg.github.io
AcidBase Titrations Ph Jump Titration See titration curves for acids and bases. The equivalence point of a titration. Which indicator would be suitable for the titration of: Titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. The successive k a 's must differ by. What are ph titration curves? This section describes what information these curves provide. Ph Jump Titration.
From mmerevise.co.uk
pH Curves Questions and Revision MME Ph Jump Titration Titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. See example 9.1 and check for understanding 9.1. In general, there are two requirements for a clearly discernible jump in the ph to occur in a polyprotic titration: Titration curves graphically represent the change in ph as titrant is added. So what we. Ph Jump Titration.
From www.youtube.com
Acid Base Titration Curves pH Calculations YouTube Ph Jump Titration See example 9.1 and check for understanding 9.1. See titration curves for acids and bases. This section describes what information these curves provide and how that information is used in chemistry. The successive k a 's must differ by. Which indicator would be suitable for the titration of: Titration is an analytical chemistry technique used to find the concentration of. Ph Jump Titration.
From saylordotorg.github.io
AcidBase Titrations Ph Jump Titration Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution. In general, there are two requirements for a clearly discernible jump in the ph to occur in a polyprotic titration: The equivalence point of a titration. Which indicator would be suitable for the titration of: So what we need is. Ph Jump Titration.
From studylib.net
Titration Curve weak base with strong acid START Ph Jump Titration The successive k a 's must differ by. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution. This section describes what information these curves provide and how that information is used in chemistry. The equivalence point of a titration. Titration curves graphically represent the change in ph as titrant. Ph Jump Titration.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Ph Jump Titration See example 9.1 and check for understanding 9.1. Which indicator would be suitable for the titration of: The equivalence point of a titration. So what we need is to find an equation where the only. The successive k a 's must differ by. This section describes what information these curves provide and how that information is used in chemistry. Titration. Ph Jump Titration.
From roqed.com
Titration (pH) curves ROQED Ph Jump Titration This section describes what information these curves provide and how that information is used in chemistry. So what we need is to find an equation where the only. Titration curves graphically represent the change in ph as titrant is added. See titration curves for acids and bases. Titration is an analytical chemistry technique used to find the concentration of an. Ph Jump Titration.
From www.youtube.com
The Titrations and pH Curves YouTube Ph Jump Titration The equivalence point of a titration. The successive k a 's must differ by. Titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. Which indicator would be suitable for the titration of: See example 9.1 and check for understanding 9.1. So what we need is to find an equation where the only.. Ph Jump Titration.
From www.slideserve.com
PPT Titration and pH Curves. PowerPoint Presentation, free download Ph Jump Titration Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution. Which indicator would be suitable for the titration of: The equivalence point of a titration. So what we need is to find an equation where the only. Titration curves graphically represent the change in ph as titrant is added. What. Ph Jump Titration.
From sansona.github.io
Titrations Ph Jump Titration Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution. In general, there are two requirements for a clearly discernible jump in the ph to occur in a polyprotic titration: Which indicator would be suitable for the titration of: Titration curves graphically represent the change in ph as titrant is. Ph Jump Titration.
From webmis.highland.cc.il.us
AcidBase Titrations Ph Jump Titration This section describes what information these curves provide and how that information is used in chemistry. Titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. Titration curves graphically represent the change in ph as titrant is added. See titration curves for acids and bases. In general, there are two requirements for a. Ph Jump Titration.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Ph Jump Titration In general, there are two requirements for a clearly discernible jump in the ph to occur in a polyprotic titration: Titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. So what we need is to find an equation where the only. See example 9.1 and check for understanding 9.1. Which indicator would. Ph Jump Titration.
From mungfali.com
Acid Base Titration Procedure Ph Jump Titration This section describes what information these curves provide and how that information is used in chemistry. See example 9.1 and check for understanding 9.1. The equivalence point of a titration. What are ph titration curves? Titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. See titration curves for acids and bases. Titration. Ph Jump Titration.
From byjus.com
Study The pH Change In The Titration Of A Strong Base Using Universal Ph Jump Titration Which indicator would be suitable for the titration of: Titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. See titration curves for acids and bases. In general, there are two requirements for a clearly discernible jump in the ph to occur in a polyprotic titration: The equivalence point of a titration. This. Ph Jump Titration.
From mmerevise.co.uk
pH Curves Questions and Revision MME Ph Jump Titration See titration curves for acids and bases. Titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. The equivalence point of a titration. Titration curves graphically represent the change in ph as titrant is added. What are ph titration curves? Which indicator would be suitable for the titration of: So what we need. Ph Jump Titration.