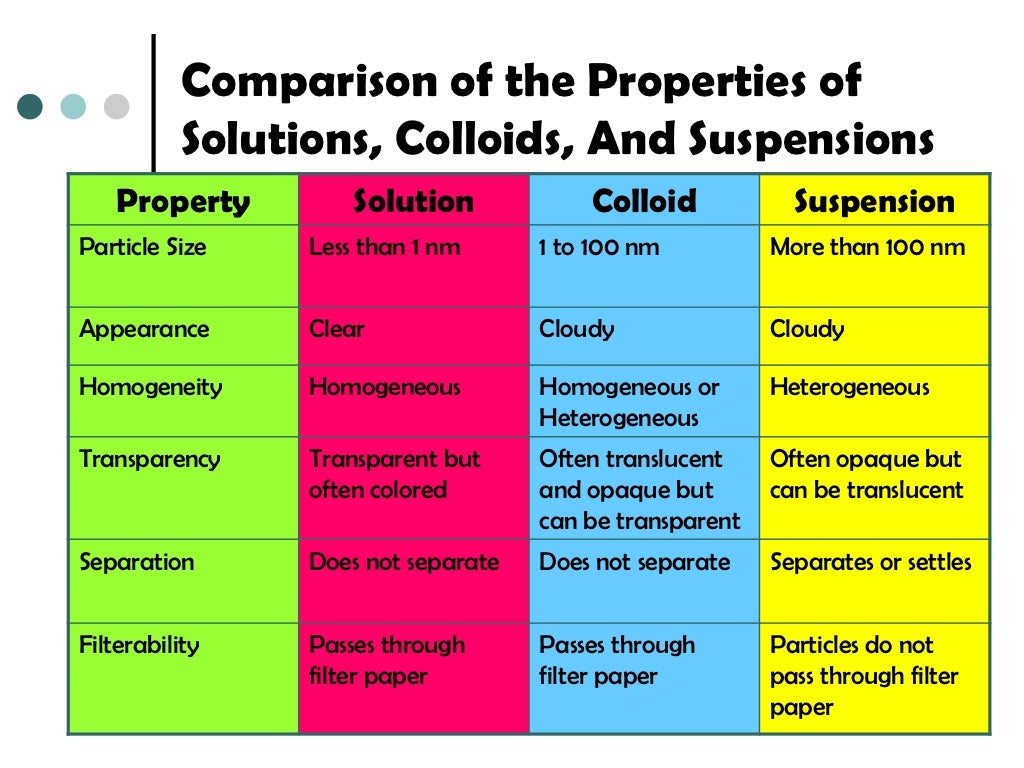
Colloid Solution Size . The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. Particles of colloidal size are formed by two methods: In a sense, they bridge the. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. Colloids occupy an intermediate place between [particulate] suspensions and solutions, both in terms of their observable properties and particle size. Particles of colloidal size are formed by two methods: A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. Colloids are unlike solutions because their dispersed particles are much larger than those of a solution. That is, by breaking down larger particles. These particles can be solid, liquid, or gas. These are also known as colloidal dispersions because the substances remain dispersed and do not settle to the bottom of the container. The dispersed particles of a colloid. A colloid is a mixture that has particles ranging between 1 and 1000 nanometers in diameter, yet are still able to remain evenly distributed throughout the solution.
from www.slideshare.net
In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. That is, by breaking down larger particles. Colloids are unlike solutions because their dispersed particles are much larger than those of a solution. A colloid is a mixture that has particles ranging between 1 and 1000 nanometers in diameter, yet are still able to remain evenly distributed throughout the solution. Particles of colloidal size are formed by two methods: Colloids occupy an intermediate place between [particulate] suspensions and solutions, both in terms of their observable properties and particle size. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. In a sense, they bridge the. Particles of colloidal size are formed by two methods:
Colloids
Colloid Solution Size A colloid is a mixture that has particles ranging between 1 and 1000 nanometers in diameter, yet are still able to remain evenly distributed throughout the solution. These are also known as colloidal dispersions because the substances remain dispersed and do not settle to the bottom of the container. A colloid is a mixture that has particles ranging between 1 and 1000 nanometers in diameter, yet are still able to remain evenly distributed throughout the solution. The dispersed particles of a colloid. Particles of colloidal size are formed by two methods: A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. Colloids are unlike solutions because their dispersed particles are much larger than those of a solution. Colloids occupy an intermediate place between [particulate] suspensions and solutions, both in terms of their observable properties and particle size. That is, by breaking down larger particles. These particles can be solid, liquid, or gas. Particles of colloidal size are formed by two methods: In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. In a sense, they bridge the.
From www.slideshare.net
Colloids Colloid Solution Size Colloids are unlike solutions because their dispersed particles are much larger than those of a solution. These particles can be solid, liquid, or gas. Particles of colloidal size are formed by two methods: In a sense, they bridge the. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. The particles are microscopic in. Colloid Solution Size.
From www.brainkart.com
Classifications of Colloidal solution Surface Chemistry Colloid Solution Size Colloids are unlike solutions because their dispersed particles are much larger than those of a solution. These particles can be solid, liquid, or gas. Particles of colloidal size are formed by two methods: Colloids occupy an intermediate place between [particulate] suspensions and solutions, both in terms of their observable properties and particle size. A colloidal solution typically consists of particles. Colloid Solution Size.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID Colloid Solution Size Colloids are unlike solutions because their dispersed particles are much larger than those of a solution. Particles of colloidal size are formed by two methods: Colloids occupy an intermediate place between [particulate] suspensions and solutions, both in terms of their observable properties and particle size. A colloid is a mixture that has particles ranging between 1 and 1000 nanometers in. Colloid Solution Size.
From www.geeksforgeeks.org
Colloids Definition, Properties, Classification & Examples Colloid Solution Size In a sense, they bridge the. These particles can be solid, liquid, or gas. Particles of colloidal size are formed by two methods: Particles of colloidal size are formed by two methods: The dispersed particles of a colloid. A colloid is a mixture that has particles ranging between 1 and 1000 nanometers in diameter, yet are still able to remain. Colloid Solution Size.
From www.slideserve.com
PPT CH110 Chapter 8 Solutions PowerPoint Presentation, free download Colloid Solution Size Colloids occupy an intermediate place between [particulate] suspensions and solutions, both in terms of their observable properties and particle size. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. The dispersed particles of a colloid. These are also known as colloidal dispersions because the substances remain dispersed and do not settle to the bottom of the. Colloid Solution Size.
From www.myshared.ru
Презентация на тему "Colloidal systems. Classes of solution True Colloid Solution Size The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. In a sense, they bridge the. Colloids occupy an intermediate place between [particulate] suspensions and solutions, both in terms of their observable properties and particle size. Particles of colloidal size are formed by two methods: Particles of colloidal size are formed by two methods: A colloidal solution. Colloid Solution Size.
From www.slideserve.com
PPT Matter Properties & Change PowerPoint Presentation, free Colloid Solution Size That is, by breaking down larger particles. Particles of colloidal size are formed by two methods: These are also known as colloidal dispersions because the substances remain dispersed and do not settle to the bottom of the container. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. Particles of colloidal size are formed by two methods:. Colloid Solution Size.
From www.sliderbase.com
Colloids and Suspensions Presentation Chemistry Colloid Solution Size Particles of colloidal size are formed by two methods: The dispersed particles of a colloid. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. Colloids occupy an intermediate place between [particulate] suspensions and solutions, both in terms of their observable properties and particle size. A colloid is a mixture that has particles ranging. Colloid Solution Size.
From byjus.com
Classification of Colloids Definition, Types, Examples, Table & Videos Colloid Solution Size Particles of colloidal size are formed by two methods: These are also known as colloidal dispersions because the substances remain dispersed and do not settle to the bottom of the container. In a sense, they bridge the. That is, by breaking down larger particles. Particles of colloidal size are formed by two methods: A colloidal solution typically consists of particles. Colloid Solution Size.
From sciencenotes.org
What Is a Colloid? Definition and Examples Colloid Solution Size Colloids occupy an intermediate place between [particulate] suspensions and solutions, both in terms of their observable properties and particle size. Colloids are unlike solutions because their dispersed particles are much larger than those of a solution. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. Particles of colloidal size are formed by two. Colloid Solution Size.
From uen.pressbooks.pub
Colloids Introductory Chemistry Colloid Solution Size A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. The dispersed particles of a colloid. In a sense, they bridge the. Particles of colloidal size are formed by two methods: Particles of colloidal size are formed by two methods: Colloids occupy an intermediate place between [particulate] suspensions and solutions, both in terms of. Colloid Solution Size.
From mungfali.com
10 Examples Of Colloids Colloid Solution Size The dispersed particles of a colloid. That is, by breaking down larger particles. A colloid is a mixture that has particles ranging between 1 and 1000 nanometers in diameter, yet are still able to remain evenly distributed throughout the solution. These are also known as colloidal dispersions because the substances remain dispersed and do not settle to the bottom of. Colloid Solution Size.
From www.geeksforgeeks.org
Suspension(Chemistry) Definition, Properties, Examples, and FAQs Colloid Solution Size A colloid is a mixture that has particles ranging between 1 and 1000 nanometers in diameter, yet are still able to remain evenly distributed throughout the solution. Colloids occupy an intermediate place between [particulate] suspensions and solutions, both in terms of their observable properties and particle size. Particles of colloidal size are formed by two methods: In chemistry, a colloid. Colloid Solution Size.
From pediaa.com
Difference Between Colloid and Solution Definition, Properties Colloid Solution Size Particles of colloidal size are formed by two methods: That is, by breaking down larger particles. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. Colloids are unlike solutions because their dispersed particles are much larger than those of a solution. Particles of colloidal size are formed by two methods: A colloidal solution. Colloid Solution Size.
From www.animalia-life.club
Examples Of Colloids Mixtures Colloid Solution Size These are also known as colloidal dispersions because the substances remain dispersed and do not settle to the bottom of the container. A colloid is a mixture that has particles ranging between 1 and 1000 nanometers in diameter, yet are still able to remain evenly distributed throughout the solution. Particles of colloidal size are formed by two methods: The particles. Colloid Solution Size.
From quizizz.com
Solution, Colloid and Suspension Science Quizizz Colloid Solution Size In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. The dispersed particles of a colloid. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. Colloids occupy an intermediate place between [particulate] suspensions and solutions, both in terms of their observable properties and particle size. That is,. Colloid Solution Size.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download Colloid Solution Size These particles can be solid, liquid, or gas. Colloids occupy an intermediate place between [particulate] suspensions and solutions, both in terms of their observable properties and particle size. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. That is, by breaking down larger particles. A colloidal solution typically consists of particles ranging in. Colloid Solution Size.
From webapi.bu.edu
Properties of colloidal solution. Colloidal System. 20221020 Colloid Solution Size A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. Colloids occupy an intermediate place between [particulate] suspensions and solutions, both in terms of their observable properties and particle size. In a sense, they bridge the. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. That is,. Colloid Solution Size.
From www.toppr.com
(k) Differentiate between solution, suspension and colloids on the Colloid Solution Size The dispersed particles of a colloid. Colloids occupy an intermediate place between [particulate] suspensions and solutions, both in terms of their observable properties and particle size. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. A colloid is a mixture that has particles ranging between 1 and 1000 nanometers in diameter, yet are. Colloid Solution Size.
From www.vecteezy.com
True Solution, Colloid solution and Suspension three different types of Colloid Solution Size These particles can be solid, liquid, or gas. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. A colloid is a mixture that has particles ranging between 1 and 1000 nanometers in diameter, yet are still able to remain evenly distributed throughout the solution. Particles of colloidal size are formed by two methods: Colloids are unlike. Colloid Solution Size.
From www.aakash.ac.in
Colloidal Solution Definition, Classification, Examples & Preparation Colloid Solution Size Colloids are unlike solutions because their dispersed particles are much larger than those of a solution. In a sense, they bridge the. These particles can be solid, liquid, or gas. Colloids occupy an intermediate place between [particulate] suspensions and solutions, both in terms of their observable properties and particle size. These are also known as colloidal dispersions because the substances. Colloid Solution Size.
From www.animalia-life.club
Examples Of Colloids Mixtures Colloid Solution Size The dispersed particles of a colloid. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. Particles of colloidal size are formed by two methods: A colloid is a mixture that has particles ranging between 1 and 1000 nanometers in diameter, yet are still able to remain evenly distributed throughout the solution. Particles of. Colloid Solution Size.
From www.sliderbase.com
Suspensions and Colloids Presentation Chemistry Colloid Solution Size A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. The dispersed particles of a colloid. These are also known as colloidal dispersions because the substances remain dispersed and do not settle to the bottom of the container. Colloids occupy an intermediate place between [particulate] suspensions and solutions, both in terms of their observable. Colloid Solution Size.
From www.animalia-life.club
Examples Of Colloids Mixtures Colloid Solution Size Colloids are unlike solutions because their dispersed particles are much larger than those of a solution. These particles can be solid, liquid, or gas. Particles of colloidal size are formed by two methods: The dispersed particles of a colloid. In a sense, they bridge the. That is, by breaking down larger particles. A colloid is a mixture that has particles. Colloid Solution Size.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download Colloid Solution Size These are also known as colloidal dispersions because the substances remain dispersed and do not settle to the bottom of the container. Particles of colloidal size are formed by two methods: A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. These particles can be solid, liquid, or gas. The dispersed particles of a. Colloid Solution Size.
From www.researchgate.net
The change of color of colloidal solution depending on size and shape Colloid Solution Size Particles of colloidal size are formed by two methods: The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. These particles can be solid, liquid, or gas. Particles of colloidal size are formed by two methods: The dispersed particles of a colloid. That is, by breaking down larger particles. These are also known as colloidal dispersions because. Colloid Solution Size.
From www.majordifferences.com
Difference Between True Solutions, Colloidal solution and Suspension Colloid Solution Size Colloids are unlike solutions because their dispersed particles are much larger than those of a solution. Colloids occupy an intermediate place between [particulate] suspensions and solutions, both in terms of their observable properties and particle size. Particles of colloidal size are formed by two methods: These are also known as colloidal dispersions because the substances remain dispersed and do not. Colloid Solution Size.
From www.logiota.com
Preparation and Properties of Colloids Surface Chemistry Physical Colloid Solution Size The dispersed particles of a colloid. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. Colloids are unlike solutions because their dispersed particles are much larger than those of a solution. In a sense, they bridge the. These particles can. Colloid Solution Size.
From www.slideserve.com
PPT Suspensions and Colloids PowerPoint Presentation, free download Colloid Solution Size The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. In a sense, they bridge the. Particles of colloidal size are formed by two methods: These particles can be solid, liquid, or gas. These are also known as colloidal dispersions because the substances remain dispersed and do not settle to the bottom of the container. A colloidal. Colloid Solution Size.
From chemwiki.ucdavis.edu
Figure 1 Examples of a stable and of an unstable colloidal dispersion Colloid Solution Size These particles can be solid, liquid, or gas. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. Colloids occupy an intermediate place between [particulate] suspensions and solutions, both in terms of their observable properties and particle size.. Colloid Solution Size.
From collegedunia.com
Classification of Colloids Properties & Phases Colloid Solution Size Particles of colloidal size are formed by two methods: In a sense, they bridge the. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. The dispersed particles of a colloid. A colloid is a mixture that has particles ranging between. Colloid Solution Size.
From www.esaral.com
Colloidal Solution Definition Examples Types for Class 12, IITJEE Colloid Solution Size These are also known as colloidal dispersions because the substances remain dispersed and do not settle to the bottom of the container. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. A colloid is a mixture that has particles ranging between 1 and 1000 nanometers in diameter, yet are still able to remain evenly distributed throughout. Colloid Solution Size.
From www.vecteezy.com
Different dispersion system, true and colloidal solution and suspension Colloid Solution Size Particles of colloidal size are formed by two methods: That is, by breaking down larger particles. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. These are also known as colloidal dispersions because the substances remain dispersed and do not settle to the bottom of the container. These particles can be solid, liquid,. Colloid Solution Size.
From www.geeksforgeeks.org
Colloids Definition, Properties, Classification & Examples Colloid Solution Size Colloids occupy an intermediate place between [particulate] suspensions and solutions, both in terms of their observable properties and particle size. Particles of colloidal size are formed by two methods: The dispersed particles of a colloid. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. In chemistry, a colloid is a mixture of tiny. Colloid Solution Size.
From www.aakash.ac.in
Colloidal Solution Definition, Classification, Examples & Preparation Colloid Solution Size These particles can be solid, liquid, or gas. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. A colloid is a mixture that has particles ranging between 1 and 1000 nanometers in diameter, yet are still able to remain evenly distributed throughout the solution. The dispersed particles of a colloid. A colloidal solution. Colloid Solution Size.