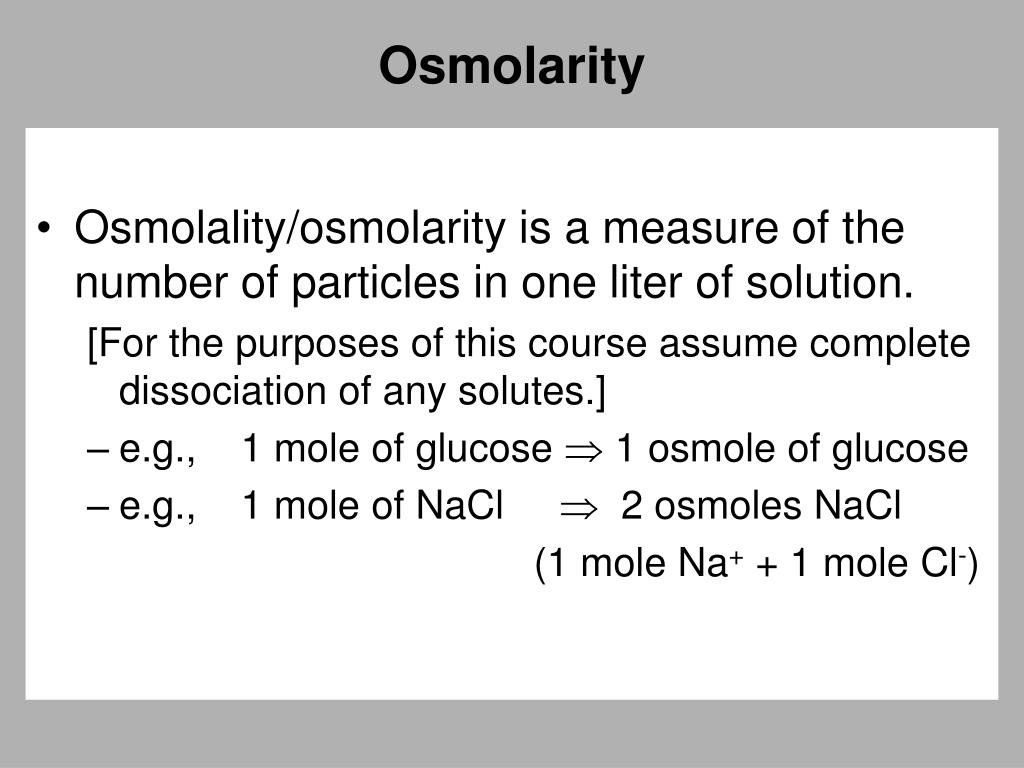
How To Measure Osmolarity . An osmole (osmol) is 1 mol of. To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. Steps required to measure the osmolarity of a solution: You multiply the molarity by the number of osmoles that each solute produces. While any polar solvent could be used, these units are used almost exclusively for aqueous (water) solutions. Now calculate the molarity of. Learn what osmolarity and osmolality are and how to express them. Calculate the moles of a solute dissolved in a solution. The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. Convert that number to osmoles per liter and add the osmolarity of each.
from www.slideserve.com
The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. While any polar solvent could be used, these units are used almost exclusively for aqueous (water) solutions. You multiply the molarity by the number of osmoles that each solute produces. Learn what osmolarity and osmolality are and how to express them. Now calculate the molarity of. Convert that number to osmoles per liter and add the osmolarity of each. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. An osmole (osmol) is 1 mol of. Calculate the moles of a solute dissolved in a solution. To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter.
PPT Diffusion PowerPoint Presentation, free download ID6798349
How To Measure Osmolarity The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. An osmole (osmol) is 1 mol of. Calculate the moles of a solute dissolved in a solution. While any polar solvent could be used, these units are used almost exclusively for aqueous (water) solutions. Convert that number to osmoles per liter and add the osmolarity of each. The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. You multiply the molarity by the number of osmoles that each solute produces. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. Learn what osmolarity and osmolality are and how to express them. Steps required to measure the osmolarity of a solution: To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. Now calculate the molarity of.
From ditki.com
Biochemistry Glossary Osmosis & Osmolarity 2. Osmolarity ditki How To Measure Osmolarity Convert that number to osmoles per liter and add the osmolarity of each. To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. Calculate the moles of a solute dissolved in a solution. While any polar solvent could be used, these units are used almost exclusively for aqueous (water) solutions. An. How To Measure Osmolarity.
From www.youtube.com
How to solve osmolarity calculation problems 4 YouTube How To Measure Osmolarity Now calculate the molarity of. Calculate the moles of a solute dissolved in a solution. Learn what osmolarity and osmolality are and how to express them. You multiply the molarity by the number of osmoles that each solute produces. An osmole (osmol) is 1 mol of. Steps required to measure the osmolarity of a solution: To calculate the osmolarity of. How To Measure Osmolarity.
From lemurianembassy.com
😍 Finding osmolarity. Calculate your own osmolarity. 20190125 How To Measure Osmolarity The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. An osmole (osmol) is 1 mol of. Convert that number to osmoles per liter and add the osmolarity of each. Learn what osmolarity and osmolality are and how to express them. To calculate the osmolarity of a solution, the first step. How To Measure Osmolarity.
From www.youtube.com
Osmolarity Example (Bio) YouTube How To Measure Osmolarity Steps required to measure the osmolarity of a solution: While any polar solvent could be used, these units are used almost exclusively for aqueous (water) solutions. Now calculate the molarity of. To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. An osmole (osmol) is 1 mol of. Learn what osmolarity. How To Measure Osmolarity.
From www.youtube.com
How to solve osmolarity calculation problems 2 YouTube How To Measure Osmolarity Calculate the moles of a solute dissolved in a solution. Now calculate the molarity of. You multiply the molarity by the number of osmoles that each solute produces. While any polar solvent could be used, these units are used almost exclusively for aqueous (water) solutions. Steps required to measure the osmolarity of a solution: To calculate the osmolarity of a. How To Measure Osmolarity.
From www.linstitute.net
IB DP Biology SL复习笔记1.3.7 Skills Estimation of Osmolarity翰林国际教育 How To Measure Osmolarity To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. An osmole (osmol) is 1 mol of. Calculate the moles of a solute dissolved in a solution. While any polar solvent could be used, these units are used almost exclusively for aqueous (water) solutions. Steps required to measure the osmolarity of. How To Measure Osmolarity.
From www.youtube.com
Calculated osmolality and osmolality gap YouTube How To Measure Osmolarity Steps required to measure the osmolarity of a solution: While any polar solvent could be used, these units are used almost exclusively for aqueous (water) solutions. Now calculate the molarity of. The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. To calculate the osmolarity of a solution, the first step. How To Measure Osmolarity.
From ditki.com
Anatomy & Physiology Osmosis and Osmolarity ditki medical How To Measure Osmolarity Calculate the moles of a solute dissolved in a solution. Steps required to measure the osmolarity of a solution: While any polar solvent could be used, these units are used almost exclusively for aqueous (water) solutions. The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. An osmole (osmol) is 1. How To Measure Osmolarity.
From www.biophlox.com
A Complete Lab Guide to Osmometer How To Measure Osmolarity You multiply the molarity by the number of osmoles that each solute produces. The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. Convert that number to osmoles per liter and add the osmolarity of each. Now calculate the molarity of. Learn what osmolarity and osmolality are and how to express. How To Measure Osmolarity.
From ibiologia.com
Osmolarity Definition, Formula & Osmolarity vs. Osmolality How To Measure Osmolarity While any polar solvent could be used, these units are used almost exclusively for aqueous (water) solutions. An osmole (osmol) is 1 mol of. Convert that number to osmoles per liter and add the osmolarity of each. Learn what osmolarity and osmolality are and how to express them. Osmolarity and osmolality are units of solute concentration that are often used. How To Measure Osmolarity.
From www.slideserve.com
PPT BODY FLUID COMPARTMENT AND FLUID BALANCE PowerPoint Presentation How To Measure Osmolarity Learn what osmolarity and osmolality are and how to express them. While any polar solvent could be used, these units are used almost exclusively for aqueous (water) solutions. Steps required to measure the osmolarity of a solution: The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. Osmolarity and osmolality are. How To Measure Osmolarity.
From www.slideserve.com
PPT ENTERAL NUTRITION PowerPoint Presentation ID225635 How To Measure Osmolarity Now calculate the molarity of. An osmole (osmol) is 1 mol of. You multiply the molarity by the number of osmoles that each solute produces. To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. Convert that number to osmoles per liter and add the osmolarity of each. Calculate the moles. How To Measure Osmolarity.
From www.youtube.com
How to Use the IPEN Tear Osmolarity Measurement System to Help with How To Measure Osmolarity The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. While any polar solvent could be used, these units are used almost exclusively for aqueous (water) solutions. Now calculate the molarity of.. How To Measure Osmolarity.
From www.pinterest.se
OSMOLARITY (serum & urine) factors that increase, factors that How To Measure Osmolarity Steps required to measure the osmolarity of a solution: Learn what osmolarity and osmolality are and how to express them. You multiply the molarity by the number of osmoles that each solute produces. The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. An osmole (osmol) is 1 mol of. Osmolarity. How To Measure Osmolarity.
From ibiologia.com
How to calculate Osmolarity from Molarity? How To Measure Osmolarity Convert that number to osmoles per liter and add the osmolarity of each. An osmole (osmol) is 1 mol of. To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. While any polar solvent could be used, these units are used almost exclusively for aqueous (water) solutions. Learn what osmolarity and. How To Measure Osmolarity.
From ditki.com
Biochemistry Glossary Osmosis & Osmolarity 1. Osmosis ditki medical How To Measure Osmolarity Convert that number to osmoles per liter and add the osmolarity of each. You multiply the molarity by the number of osmoles that each solute produces. Steps required to measure the osmolarity of a solution: An osmole (osmol) is 1 mol of. Learn what osmolarity and osmolality are and how to express them. Osmolarity and osmolality are units of solute. How To Measure Osmolarity.
From www.youtube.com
Estimating Serum Osmolality using a Simple Formula YouTube How To Measure Osmolarity Learn what osmolarity and osmolality are and how to express them. You multiply the molarity by the number of osmoles that each solute produces. Now calculate the molarity of. The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. Osmolarity and osmolality are units of solute concentration that are often used. How To Measure Osmolarity.
From www.youtube.com
Chemistry Basics Osmolarity, Osmolality and Tonicity YouTube How To Measure Osmolarity Now calculate the molarity of. Convert that number to osmoles per liter and add the osmolarity of each. The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. Learn what osmolarity and osmolality are and how to express them. While any polar solvent could be used, these units are used almost. How To Measure Osmolarity.
From www.thetechedvocate.org
How to calculate osmolarity The Tech Edvocate How To Measure Osmolarity Steps required to measure the osmolarity of a solution: Now calculate the molarity of. Convert that number to osmoles per liter and add the osmolarity of each. To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. Osmolarity and osmolality are units of solute concentration that are often used in reference. How To Measure Osmolarity.
From clinicalsci.info
Measurement of Urine and Plasma Osmolality » Clinical Laboratory Science How To Measure Osmolarity Steps required to measure the osmolarity of a solution: An osmole (osmol) is 1 mol of. To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. Calculate the moles of a solute. How To Measure Osmolarity.
From study.com
Serum Osmolality Definition, Calculation & Interpretation Lesson How To Measure Osmolarity Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. Steps required to measure the osmolarity of a solution: To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. Convert that number to osmoles per liter and add the osmolarity of each.. How To Measure Osmolarity.
From www.delawareeyecare.com
TearLab Osmolarity System Delaware Eye Care Center How To Measure Osmolarity Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. An osmole (osmol) is 1 mol of. Learn what osmolarity and osmolality are and how to express them. Convert that number to osmoles per liter and add the osmolarity of each. Now calculate the molarity of. The salts of sodium (choloride. How To Measure Osmolarity.
From www.slideserve.com
PPT PHYSICAL EXAMINATION OF URINE PowerPoint Presentation ID7036624 How To Measure Osmolarity Learn what osmolarity and osmolality are and how to express them. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. An osmole (osmol) is 1 mol of. To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. The salts of sodium. How To Measure Osmolarity.
From ojklmexhbr.blogspot.com
How To Calculate Serum Osmolality In this study, we compared the How To Measure Osmolarity To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. You multiply the molarity by the number of osmoles that each solute produces. Steps required to measure the osmolarity of a solution:. How To Measure Osmolarity.
From www.youtube.com
Osmolarity Calculations Review 5 Key Examples Solved YouTube How To Measure Osmolarity To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. While any polar solvent could be used, these units are used almost exclusively for aqueous (water) solutions. The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. Osmolarity and osmolality are units. How To Measure Osmolarity.
From www.youtube.com
1.4 Skill Estimation of osmolarity in tissues (Practical 2) YouTube How To Measure Osmolarity Steps required to measure the osmolarity of a solution: Now calculate the molarity of. Learn what osmolarity and osmolality are and how to express them. You multiply the molarity by the number of osmoles that each solute produces. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. To calculate the. How To Measure Osmolarity.
From www.youtube.com
Calculated Osmolality YouTube How To Measure Osmolarity Steps required to measure the osmolarity of a solution: You multiply the molarity by the number of osmoles that each solute produces. The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter.. How To Measure Osmolarity.
From www.youtube.com
Renal system 13 Osmolarity How to calculate osmotic pressure How To Measure Osmolarity Calculate the moles of a solute dissolved in a solution. While any polar solvent could be used, these units are used almost exclusively for aqueous (water) solutions. Convert that number to osmoles per liter and add the osmolarity of each. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. To. How To Measure Osmolarity.
From nikolausalexa.blogspot.com
31+ solution osmolarity calculator NikolausAlexa How To Measure Osmolarity To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. Now calculate the molarity of. The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. An osmole (osmol) is 1 mol of. You multiply the molarity by the number of osmoles that. How To Measure Osmolarity.
From www.pinterest.ca
Urine and Blood Osmolality Fishbone Cheat Sheet Mnemonic Nursing How To Measure Osmolarity Steps required to measure the osmolarity of a solution: The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. Learn what osmolarity and osmolality are and how to express them. To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. You multiply. How To Measure Osmolarity.
From www.slideserve.com
PPT Diffusion PowerPoint Presentation, free download ID6798349 How To Measure Osmolarity An osmole (osmol) is 1 mol of. You multiply the molarity by the number of osmoles that each solute produces. Now calculate the molarity of. While any polar solvent could be used, these units are used almost exclusively for aqueous (water) solutions. Calculate the moles of a solute dissolved in a solution. Steps required to measure the osmolarity of a. How To Measure Osmolarity.
From ojklmexhbr.blogspot.com
How To Calculate Serum Osmolality In this study, we compared the How To Measure Osmolarity Now calculate the molarity of. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. Calculate the moles of a solute dissolved in a solution. The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. An osmole (osmol) is 1 mol of.. How To Measure Osmolarity.
From www.youtube.com
Calculating the Osmolarity of a Solution YouTube How To Measure Osmolarity The salts of sodium (choloride and bicarbonate) and nonelectrolyte glucose and urea are the major five osmoles of plasma. To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. An osmole (osmol) is 1 mol of. While any polar solvent could be used, these units are used almost exclusively for aqueous. How To Measure Osmolarity.
From www.youtube.com
How to solve osmolarity calculation problems 3 YouTube How To Measure Osmolarity Convert that number to osmoles per liter and add the osmolarity of each. Now calculate the molarity of. While any polar solvent could be used, these units are used almost exclusively for aqueous (water) solutions. Calculate the moles of a solute dissolved in a solution. Steps required to measure the osmolarity of a solution: You multiply the molarity by the. How To Measure Osmolarity.
From www.youtube.com
Calculating the Osmolarity of a Solution (Question 2) YouTube How To Measure Osmolarity Learn what osmolarity and osmolality are and how to express them. Steps required to measure the osmolarity of a solution: Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. While any polar solvent could be used, these units are used almost exclusively for aqueous (water) solutions. To calculate the osmolarity. How To Measure Osmolarity.