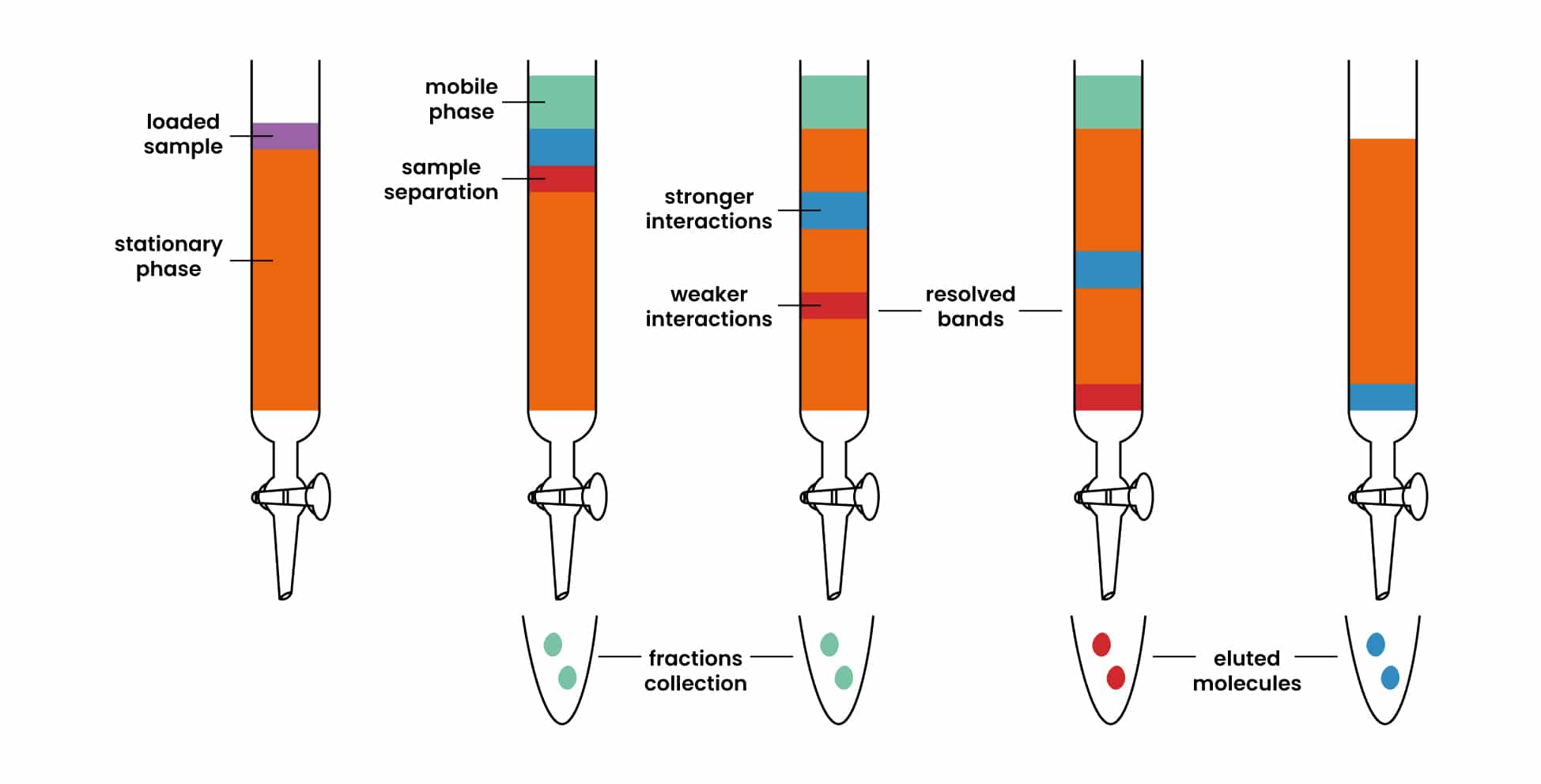
Is Chromatography Solvent Polar Or Nonpolar . Why is it important to use a nonpolar solvent (such as hexane, acetone and trichloromethane) and not a polar solvent (such as water) to investigate plant pigments. In almost all applications of tlc, the stationary phase is a silica or alumina adsorbent and the. By adjusting the composition of the mobile phase, the migration rate can be controlled, leading to efficient separation. The solvent may be polar (like water), somewhat polar (like isopropyl alcohol), or nonpolar (like vegetable oil). The mobile phase can be isocratic or gradient, polar. For this example, let’s say our solvent is nonpolar, and we are using. The solvent used for chromatography will be selected based on the polarity of the substances in the mixture you. In all forms of chromatography, samples equilibrate between stationary and mobile phases.
from bitesizebio.com
Why is it important to use a nonpolar solvent (such as hexane, acetone and trichloromethane) and not a polar solvent (such as water) to investigate plant pigments. In all forms of chromatography, samples equilibrate between stationary and mobile phases. The solvent may be polar (like water), somewhat polar (like isopropyl alcohol), or nonpolar (like vegetable oil). The mobile phase can be isocratic or gradient, polar. For this example, let’s say our solvent is nonpolar, and we are using. By adjusting the composition of the mobile phase, the migration rate can be controlled, leading to efficient separation. In almost all applications of tlc, the stationary phase is a silica or alumina adsorbent and the. The solvent used for chromatography will be selected based on the polarity of the substances in the mixture you.
Column Chromatography Made Simple An Easy to Follow Guide
Is Chromatography Solvent Polar Or Nonpolar In all forms of chromatography, samples equilibrate between stationary and mobile phases. By adjusting the composition of the mobile phase, the migration rate can be controlled, leading to efficient separation. The mobile phase can be isocratic or gradient, polar. In almost all applications of tlc, the stationary phase is a silica or alumina adsorbent and the. Why is it important to use a nonpolar solvent (such as hexane, acetone and trichloromethane) and not a polar solvent (such as water) to investigate plant pigments. The solvent used for chromatography will be selected based on the polarity of the substances in the mixture you. For this example, let’s say our solvent is nonpolar, and we are using. In all forms of chromatography, samples equilibrate between stationary and mobile phases. The solvent may be polar (like water), somewhat polar (like isopropyl alcohol), or nonpolar (like vegetable oil).
From bitesizebio.com
Column Chromatography Made Simple An Easy to Follow Guide Is Chromatography Solvent Polar Or Nonpolar By adjusting the composition of the mobile phase, the migration rate can be controlled, leading to efficient separation. The mobile phase can be isocratic or gradient, polar. In almost all applications of tlc, the stationary phase is a silica or alumina adsorbent and the. For this example, let’s say our solvent is nonpolar, and we are using. Why is it. Is Chromatography Solvent Polar Or Nonpolar.
From lab-training.com
Gas Chromatography Principles, Types and Working Is Chromatography Solvent Polar Or Nonpolar The solvent used for chromatography will be selected based on the polarity of the substances in the mixture you. Why is it important to use a nonpolar solvent (such as hexane, acetone and trichloromethane) and not a polar solvent (such as water) to investigate plant pigments. In almost all applications of tlc, the stationary phase is a silica or alumina. Is Chromatography Solvent Polar Or Nonpolar.
From mavink.com
Chromatography Diagram Labeled Is Chromatography Solvent Polar Or Nonpolar The solvent may be polar (like water), somewhat polar (like isopropyl alcohol), or nonpolar (like vegetable oil). The mobile phase can be isocratic or gradient, polar. In all forms of chromatography, samples equilibrate between stationary and mobile phases. By adjusting the composition of the mobile phase, the migration rate can be controlled, leading to efficient separation. In almost all applications. Is Chromatography Solvent Polar Or Nonpolar.
From byjus.com
What is Polar and Nonpolar Solvent? Is Chromatography Solvent Polar Or Nonpolar The solvent may be polar (like water), somewhat polar (like isopropyl alcohol), or nonpolar (like vegetable oil). The mobile phase can be isocratic or gradient, polar. The solvent used for chromatography will be selected based on the polarity of the substances in the mixture you. By adjusting the composition of the mobile phase, the migration rate can be controlled, leading. Is Chromatography Solvent Polar Or Nonpolar.
From divorcereferred.gq
Solvent used in chromatography \ Hormoner Is Chromatography Solvent Polar Or Nonpolar In almost all applications of tlc, the stationary phase is a silica or alumina adsorbent and the. The mobile phase can be isocratic or gradient, polar. By adjusting the composition of the mobile phase, the migration rate can be controlled, leading to efficient separation. In all forms of chromatography, samples equilibrate between stationary and mobile phases. For this example, let’s. Is Chromatography Solvent Polar Or Nonpolar.
From emerypharma.com
Chromatography is Full of Ups and Downs! Is Chromatography Solvent Polar Or Nonpolar The mobile phase can be isocratic or gradient, polar. In almost all applications of tlc, the stationary phase is a silica or alumina adsorbent and the. The solvent may be polar (like water), somewhat polar (like isopropyl alcohol), or nonpolar (like vegetable oil). For this example, let’s say our solvent is nonpolar, and we are using. In all forms of. Is Chromatography Solvent Polar Or Nonpolar.
From www.slideserve.com
PPT Chapter 12 SOLUTIONS PowerPoint Presentation, free download ID Is Chromatography Solvent Polar Or Nonpolar The mobile phase can be isocratic or gradient, polar. In all forms of chromatography, samples equilibrate between stationary and mobile phases. By adjusting the composition of the mobile phase, the migration rate can be controlled, leading to efficient separation. In almost all applications of tlc, the stationary phase is a silica or alumina adsorbent and the. Why is it important. Is Chromatography Solvent Polar Or Nonpolar.
From blog.sepscience.com
Basis of Interactions in Gas Chromatography, Part 1 NonPolar Is Chromatography Solvent Polar Or Nonpolar In almost all applications of tlc, the stationary phase is a silica or alumina adsorbent and the. By adjusting the composition of the mobile phase, the migration rate can be controlled, leading to efficient separation. For this example, let’s say our solvent is nonpolar, and we are using. The solvent may be polar (like water), somewhat polar (like isopropyl alcohol),. Is Chromatography Solvent Polar Or Nonpolar.
From owlcation.com
What Is Paper Chromatography and How Does it Work? Owlcation Is Chromatography Solvent Polar Or Nonpolar By adjusting the composition of the mobile phase, the migration rate can be controlled, leading to efficient separation. The solvent used for chromatography will be selected based on the polarity of the substances in the mixture you. The mobile phase can be isocratic or gradient, polar. The solvent may be polar (like water), somewhat polar (like isopropyl alcohol), or nonpolar. Is Chromatography Solvent Polar Or Nonpolar.
From jackwestin.com
Chromatography Basic Principles Involved In Separation Process Is Chromatography Solvent Polar Or Nonpolar Why is it important to use a nonpolar solvent (such as hexane, acetone and trichloromethane) and not a polar solvent (such as water) to investigate plant pigments. The solvent may be polar (like water), somewhat polar (like isopropyl alcohol), or nonpolar (like vegetable oil). In all forms of chromatography, samples equilibrate between stationary and mobile phases. In almost all applications. Is Chromatography Solvent Polar Or Nonpolar.
From slidetodoc.com
Colors in Chemistry Learning Polarity with Paper Chromatography Is Chromatography Solvent Polar Or Nonpolar For this example, let’s say our solvent is nonpolar, and we are using. The solvent used for chromatography will be selected based on the polarity of the substances in the mixture you. In all forms of chromatography, samples equilibrate between stationary and mobile phases. By adjusting the composition of the mobile phase, the migration rate can be controlled, leading to. Is Chromatography Solvent Polar Or Nonpolar.
From www.slideserve.com
PPT Chapter 8 ThinLayer Chromatography PowerPoint Presentation, free Is Chromatography Solvent Polar Or Nonpolar For this example, let’s say our solvent is nonpolar, and we are using. The mobile phase can be isocratic or gradient, polar. By adjusting the composition of the mobile phase, the migration rate can be controlled, leading to efficient separation. In all forms of chromatography, samples equilibrate between stationary and mobile phases. Why is it important to use a nonpolar. Is Chromatography Solvent Polar Or Nonpolar.
From slideplayer.com
High performance liquid chromatograph ppt download Is Chromatography Solvent Polar Or Nonpolar By adjusting the composition of the mobile phase, the migration rate can be controlled, leading to efficient separation. The mobile phase can be isocratic or gradient, polar. For this example, let’s say our solvent is nonpolar, and we are using. In all forms of chromatography, samples equilibrate between stationary and mobile phases. In almost all applications of tlc, the stationary. Is Chromatography Solvent Polar Or Nonpolar.
From stilleducation.com
Polar and Nonpolar Covalent Bonds Characteristics & Differences Is Chromatography Solvent Polar Or Nonpolar In all forms of chromatography, samples equilibrate between stationary and mobile phases. By adjusting the composition of the mobile phase, the migration rate can be controlled, leading to efficient separation. The solvent may be polar (like water), somewhat polar (like isopropyl alcohol), or nonpolar (like vegetable oil). Why is it important to use a nonpolar solvent (such as hexane, acetone. Is Chromatography Solvent Polar Or Nonpolar.
From slidetodoc.com
PolarNonpolar solvents using chromatography Purpose To use paper Is Chromatography Solvent Polar Or Nonpolar The mobile phase can be isocratic or gradient, polar. The solvent used for chromatography will be selected based on the polarity of the substances in the mixture you. Why is it important to use a nonpolar solvent (such as hexane, acetone and trichloromethane) and not a polar solvent (such as water) to investigate plant pigments. For this example, let’s say. Is Chromatography Solvent Polar Or Nonpolar.
From www.teachoo.com
What are the Properties of Mixtures? Teachoo Concepts Is Chromatography Solvent Polar Or Nonpolar The solvent used for chromatography will be selected based on the polarity of the substances in the mixture you. In almost all applications of tlc, the stationary phase is a silica or alumina adsorbent and the. In all forms of chromatography, samples equilibrate between stationary and mobile phases. Why is it important to use a nonpolar solvent (such as hexane,. Is Chromatography Solvent Polar Or Nonpolar.
From pediaa.com
Difference Between Polar and Nonpolar Molecules Definition, Formation Is Chromatography Solvent Polar Or Nonpolar In almost all applications of tlc, the stationary phase is a silica or alumina adsorbent and the. By adjusting the composition of the mobile phase, the migration rate can be controlled, leading to efficient separation. The solvent used for chromatography will be selected based on the polarity of the substances in the mixture you. For this example, let’s say our. Is Chromatography Solvent Polar Or Nonpolar.
From www.youtube.com
Selection of solvent in chromatography column chromatography Is Chromatography Solvent Polar Or Nonpolar The solvent may be polar (like water), somewhat polar (like isopropyl alcohol), or nonpolar (like vegetable oil). For this example, let’s say our solvent is nonpolar, and we are using. Why is it important to use a nonpolar solvent (such as hexane, acetone and trichloromethane) and not a polar solvent (such as water) to investigate plant pigments. The solvent used. Is Chromatography Solvent Polar Or Nonpolar.
From www.makingmolecules.com
Thin Layer Chromatography (TLC) — Making Molecules Is Chromatography Solvent Polar Or Nonpolar In all forms of chromatography, samples equilibrate between stationary and mobile phases. The mobile phase can be isocratic or gradient, polar. By adjusting the composition of the mobile phase, the migration rate can be controlled, leading to efficient separation. For this example, let’s say our solvent is nonpolar, and we are using. The solvent may be polar (like water), somewhat. Is Chromatography Solvent Polar Or Nonpolar.
From mungfali.com
Solvent Polarity Chart Is Chromatography Solvent Polar Or Nonpolar The solvent may be polar (like water), somewhat polar (like isopropyl alcohol), or nonpolar (like vegetable oil). In all forms of chromatography, samples equilibrate between stationary and mobile phases. For this example, let’s say our solvent is nonpolar, and we are using. By adjusting the composition of the mobile phase, the migration rate can be controlled, leading to efficient separation.. Is Chromatography Solvent Polar Or Nonpolar.
From studylib.net
Polar and Nonpolar Molecules Is Chromatography Solvent Polar Or Nonpolar The mobile phase can be isocratic or gradient, polar. The solvent may be polar (like water), somewhat polar (like isopropyl alcohol), or nonpolar (like vegetable oil). In all forms of chromatography, samples equilibrate between stationary and mobile phases. By adjusting the composition of the mobile phase, the migration rate can be controlled, leading to efficient separation. For this example, let’s. Is Chromatography Solvent Polar Or Nonpolar.
From mavink.com
Thin Layer Chromatography Polarity Is Chromatography Solvent Polar Or Nonpolar In all forms of chromatography, samples equilibrate between stationary and mobile phases. The solvent may be polar (like water), somewhat polar (like isopropyl alcohol), or nonpolar (like vegetable oil). The mobile phase can be isocratic or gradient, polar. For this example, let’s say our solvent is nonpolar, and we are using. Why is it important to use a nonpolar solvent. Is Chromatography Solvent Polar Or Nonpolar.
From www.differencebetween.com
Difference Between Polar and Nonpolar Solvents Compare the Difference Is Chromatography Solvent Polar Or Nonpolar In almost all applications of tlc, the stationary phase is a silica or alumina adsorbent and the. In all forms of chromatography, samples equilibrate between stationary and mobile phases. Why is it important to use a nonpolar solvent (such as hexane, acetone and trichloromethane) and not a polar solvent (such as water) to investigate plant pigments. For this example, let’s. Is Chromatography Solvent Polar Or Nonpolar.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID269800 Is Chromatography Solvent Polar Or Nonpolar In all forms of chromatography, samples equilibrate between stationary and mobile phases. In almost all applications of tlc, the stationary phase is a silica or alumina adsorbent and the. The solvent used for chromatography will be selected based on the polarity of the substances in the mixture you. For this example, let’s say our solvent is nonpolar, and we are. Is Chromatography Solvent Polar Or Nonpolar.
From simp-link.com
Difference between polar and nonpolar examples Is Chromatography Solvent Polar Or Nonpolar In almost all applications of tlc, the stationary phase is a silica or alumina adsorbent and the. The solvent may be polar (like water), somewhat polar (like isopropyl alcohol), or nonpolar (like vegetable oil). Why is it important to use a nonpolar solvent (such as hexane, acetone and trichloromethane) and not a polar solvent (such as water) to investigate plant. Is Chromatography Solvent Polar Or Nonpolar.
From www.masterorganicchemistry.com
Polar Protic? Polar Aprotic? Nonpolar? All About Solvents Master Is Chromatography Solvent Polar Or Nonpolar The mobile phase can be isocratic or gradient, polar. The solvent may be polar (like water), somewhat polar (like isopropyl alcohol), or nonpolar (like vegetable oil). The solvent used for chromatography will be selected based on the polarity of the substances in the mixture you. In all forms of chromatography, samples equilibrate between stationary and mobile phases. Why is it. Is Chromatography Solvent Polar Or Nonpolar.
From www.numerade.com
SOLVED Student Activity Chromatography Separation of Photopigments Is Chromatography Solvent Polar Or Nonpolar In almost all applications of tlc, the stationary phase is a silica or alumina adsorbent and the. In all forms of chromatography, samples equilibrate between stationary and mobile phases. Why is it important to use a nonpolar solvent (such as hexane, acetone and trichloromethane) and not a polar solvent (such as water) to investigate plant pigments. For this example, let’s. Is Chromatography Solvent Polar Or Nonpolar.
From www.yaclass.in
Chromatography — lesson. Science CBSE, Class 9. Is Chromatography Solvent Polar Or Nonpolar In all forms of chromatography, samples equilibrate between stationary and mobile phases. The mobile phase can be isocratic or gradient, polar. The solvent may be polar (like water), somewhat polar (like isopropyl alcohol), or nonpolar (like vegetable oil). Why is it important to use a nonpolar solvent (such as hexane, acetone and trichloromethane) and not a polar solvent (such as. Is Chromatography Solvent Polar Or Nonpolar.
From alexmistry.z13.web.core.windows.net
Polarity Of Solvents In Chromatography Is Chromatography Solvent Polar Or Nonpolar Why is it important to use a nonpolar solvent (such as hexane, acetone and trichloromethane) and not a polar solvent (such as water) to investigate plant pigments. In almost all applications of tlc, the stationary phase is a silica or alumina adsorbent and the. In all forms of chromatography, samples equilibrate between stationary and mobile phases. For this example, let’s. Is Chromatography Solvent Polar Or Nonpolar.
From sonataclub.jodymaroni.com
Chromatography Definition, Principles, Types, Applications, FAQs Is Chromatography Solvent Polar Or Nonpolar For this example, let’s say our solvent is nonpolar, and we are using. In almost all applications of tlc, the stationary phase is a silica or alumina adsorbent and the. By adjusting the composition of the mobile phase, the migration rate can be controlled, leading to efficient separation. The solvent may be polar (like water), somewhat polar (like isopropyl alcohol),. Is Chromatography Solvent Polar Or Nonpolar.
From sciencenotes.org
Polar and Nonpolar Molecules Is Chromatography Solvent Polar Or Nonpolar The solvent used for chromatography will be selected based on the polarity of the substances in the mixture you. In almost all applications of tlc, the stationary phase is a silica or alumina adsorbent and the. The mobile phase can be isocratic or gradient, polar. By adjusting the composition of the mobile phase, the migration rate can be controlled, leading. Is Chromatography Solvent Polar Or Nonpolar.
From www.researchgate.net
Screening of polar and nonpolar solvents to optimise the reaction Is Chromatography Solvent Polar Or Nonpolar For this example, let’s say our solvent is nonpolar, and we are using. The solvent used for chromatography will be selected based on the polarity of the substances in the mixture you. In all forms of chromatography, samples equilibrate between stationary and mobile phases. By adjusting the composition of the mobile phase, the migration rate can be controlled, leading to. Is Chromatography Solvent Polar Or Nonpolar.
From www.learnatnoon.com
What is Chromatography? Noon Academy Is Chromatography Solvent Polar Or Nonpolar The mobile phase can be isocratic or gradient, polar. In all forms of chromatography, samples equilibrate between stationary and mobile phases. The solvent used for chromatography will be selected based on the polarity of the substances in the mixture you. By adjusting the composition of the mobile phase, the migration rate can be controlled, leading to efficient separation. In almost. Is Chromatography Solvent Polar Or Nonpolar.
From www.slideserve.com
PPT classification of chromatography PowerPoint Presentation, free Is Chromatography Solvent Polar Or Nonpolar For this example, let’s say our solvent is nonpolar, and we are using. The solvent used for chromatography will be selected based on the polarity of the substances in the mixture you. Why is it important to use a nonpolar solvent (such as hexane, acetone and trichloromethane) and not a polar solvent (such as water) to investigate plant pigments. In. Is Chromatography Solvent Polar Or Nonpolar.
From www.slideserve.com
PPT Chapter 2 Chemistry PowerPoint Presentation, free Is Chromatography Solvent Polar Or Nonpolar For this example, let’s say our solvent is nonpolar, and we are using. The solvent may be polar (like water), somewhat polar (like isopropyl alcohol), or nonpolar (like vegetable oil). The solvent used for chromatography will be selected based on the polarity of the substances in the mixture you. In almost all applications of tlc, the stationary phase is a. Is Chromatography Solvent Polar Or Nonpolar.