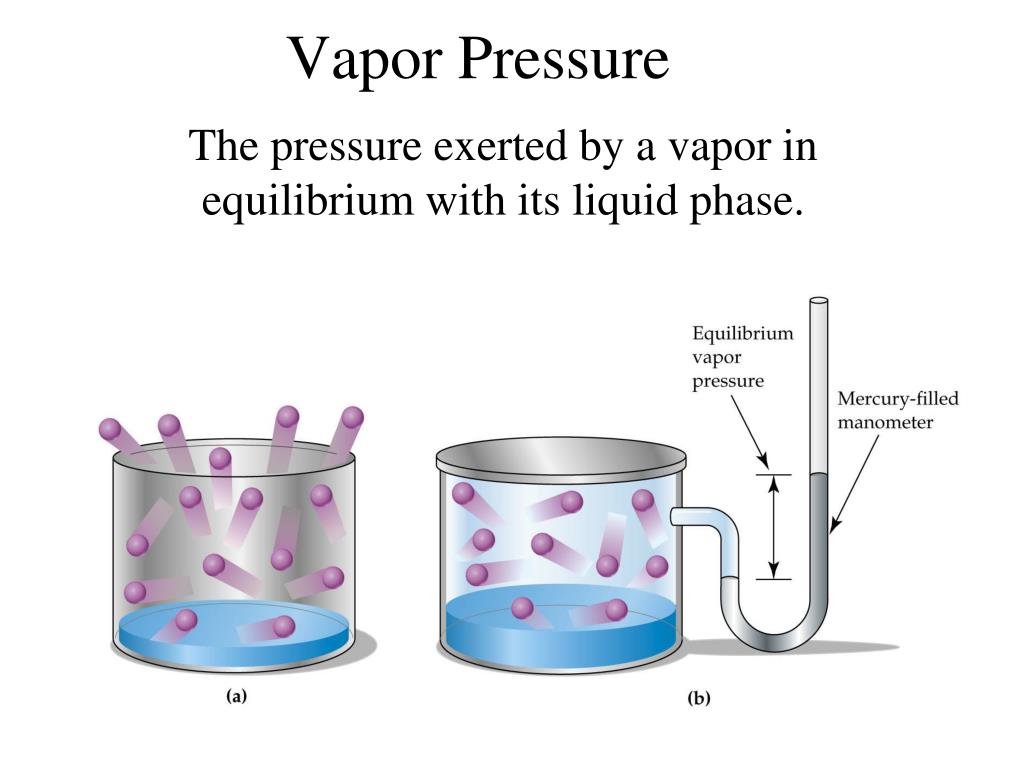
What Causes Water's Low Vapor Pressure Quizlet . The line on the graph shows the boiling temperature for water. The vapour pressure of water is depressed by the degree of intermolecular interaction (intermolecular force) present in. Vapor pressure is defined as the partial pressure of a substance in the gas phase (vapor) that exists above a sample of the liquid in a closed container. The phenomenon of vapor pressure. Nonvolatile liquids have low vapor pressures. As the temperature of a. What causes water's low vapor pressure? When the vapor pressure equals the. Study with quizlet and memorize flashcards containing terms like how does hydrogen bonding affect water molecules?, what causes water's. Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; The vapor pressure of a liquid varies with its temperature, as the following graph shows for water.
from www.slideserve.com
As the temperature of a. Study with quizlet and memorize flashcards containing terms like how does hydrogen bonding affect water molecules?, what causes water's. The vapour pressure of water is depressed by the degree of intermolecular interaction (intermolecular force) present in. Nonvolatile liquids have low vapor pressures. What causes water's low vapor pressure? Vapor pressure is defined as the partial pressure of a substance in the gas phase (vapor) that exists above a sample of the liquid in a closed container. Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; The phenomenon of vapor pressure. The vapor pressure of a liquid varies with its temperature, as the following graph shows for water. The line on the graph shows the boiling temperature for water.
PPT Liquids, Solids, and Intermolecular Forces PowerPoint
What Causes Water's Low Vapor Pressure Quizlet When the vapor pressure equals the. Vapor pressure is defined as the partial pressure of a substance in the gas phase (vapor) that exists above a sample of the liquid in a closed container. As the temperature of a. What causes water's low vapor pressure? The vapor pressure of a liquid varies with its temperature, as the following graph shows for water. The vapour pressure of water is depressed by the degree of intermolecular interaction (intermolecular force) present in. Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; Study with quizlet and memorize flashcards containing terms like how does hydrogen bonding affect water molecules?, what causes water's. When the vapor pressure equals the. Nonvolatile liquids have low vapor pressures. The line on the graph shows the boiling temperature for water. The phenomenon of vapor pressure.
From www.youtube.com
Colligative properties Vapor pressure lowering YouTube What Causes Water's Low Vapor Pressure Quizlet When the vapor pressure equals the. The vapor pressure of a liquid varies with its temperature, as the following graph shows for water. Nonvolatile liquids have low vapor pressures. The vapour pressure of water is depressed by the degree of intermolecular interaction (intermolecular force) present in. The phenomenon of vapor pressure. Volatile liquids are liquids with high vapor pressures, which. What Causes Water's Low Vapor Pressure Quizlet.
From chemyam.blogspot.com
COLLIGATIVE PROPERTIES (Relative lowering of vapour pressure) What Causes Water's Low Vapor Pressure Quizlet Nonvolatile liquids have low vapor pressures. The vapor pressure of a liquid varies with its temperature, as the following graph shows for water. What causes water's low vapor pressure? Study with quizlet and memorize flashcards containing terms like how does hydrogen bonding affect water molecules?, what causes water's. As the temperature of a. Volatile liquids are liquids with high vapor. What Causes Water's Low Vapor Pressure Quizlet.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID7038735 What Causes Water's Low Vapor Pressure Quizlet As the temperature of a. Vapor pressure is defined as the partial pressure of a substance in the gas phase (vapor) that exists above a sample of the liquid in a closed container. Nonvolatile liquids have low vapor pressures. The vapor pressure of a liquid varies with its temperature, as the following graph shows for water. The phenomenon of vapor. What Causes Water's Low Vapor Pressure Quizlet.
From www.youtube.com
Vapor Pressure and Boiling YouTube What Causes Water's Low Vapor Pressure Quizlet Vapor pressure is defined as the partial pressure of a substance in the gas phase (vapor) that exists above a sample of the liquid in a closed container. The vapor pressure of a liquid varies with its temperature, as the following graph shows for water. What causes water's low vapor pressure? The vapour pressure of water is depressed by the. What Causes Water's Low Vapor Pressure Quizlet.
From www.youtube.com
Which Compound Has a Higher Vapor Pressure? Intermolecular Force What Causes Water's Low Vapor Pressure Quizlet When the vapor pressure equals the. The vapour pressure of water is depressed by the degree of intermolecular interaction (intermolecular force) present in. The vapor pressure of a liquid varies with its temperature, as the following graph shows for water. The phenomenon of vapor pressure. The line on the graph shows the boiling temperature for water. As the temperature of. What Causes Water's Low Vapor Pressure Quizlet.
From joibqralp.blob.core.windows.net
Vapor Water Cycle Definition at Ashlee Thompson blog What Causes Water's Low Vapor Pressure Quizlet The vapour pressure of water is depressed by the degree of intermolecular interaction (intermolecular force) present in. When the vapor pressure equals the. Vapor pressure is defined as the partial pressure of a substance in the gas phase (vapor) that exists above a sample of the liquid in a closed container. The vapor pressure of a liquid varies with its. What Causes Water's Low Vapor Pressure Quizlet.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps What Causes Water's Low Vapor Pressure Quizlet Vapor pressure is defined as the partial pressure of a substance in the gas phase (vapor) that exists above a sample of the liquid in a closed container. Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; The line on the graph shows the boiling temperature for water. As the temperature of. What Causes Water's Low Vapor Pressure Quizlet.
From mungfali.com
Water Vapor Pressure Temperature Chart What Causes Water's Low Vapor Pressure Quizlet Study with quizlet and memorize flashcards containing terms like how does hydrogen bonding affect water molecules?, what causes water's. As the temperature of a. The vapour pressure of water is depressed by the degree of intermolecular interaction (intermolecular force) present in. The line on the graph shows the boiling temperature for water. Volatile liquids are liquids with high vapor pressures,. What Causes Water's Low Vapor Pressure Quizlet.
From pediaa.com
Difference Between Vapor Pressure and Boiling Point Definition What Causes Water's Low Vapor Pressure Quizlet Study with quizlet and memorize flashcards containing terms like how does hydrogen bonding affect water molecules?, what causes water's. Nonvolatile liquids have low vapor pressures. Vapor pressure is defined as the partial pressure of a substance in the gas phase (vapor) that exists above a sample of the liquid in a closed container. What causes water's low vapor pressure? The. What Causes Water's Low Vapor Pressure Quizlet.
From www.slideserve.com
PPT Solution properties PowerPoint Presentation, free download ID What Causes Water's Low Vapor Pressure Quizlet What causes water's low vapor pressure? Vapor pressure is defined as the partial pressure of a substance in the gas phase (vapor) that exists above a sample of the liquid in a closed container. The line on the graph shows the boiling temperature for water. When the vapor pressure equals the. Nonvolatile liquids have low vapor pressures. Volatile liquids are. What Causes Water's Low Vapor Pressure Quizlet.
From techblog.ctgclean.com
Drying Vapor Pressure vs. Temperature CTG Clean What Causes Water's Low Vapor Pressure Quizlet The phenomenon of vapor pressure. When the vapor pressure equals the. Nonvolatile liquids have low vapor pressures. The vapor pressure of a liquid varies with its temperature, as the following graph shows for water. As the temperature of a. What causes water's low vapor pressure? Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an. What Causes Water's Low Vapor Pressure Quizlet.
From www.slideserve.com
PPT Why . . . PowerPoint Presentation, free download ID2250075 What Causes Water's Low Vapor Pressure Quizlet Vapor pressure is defined as the partial pressure of a substance in the gas phase (vapor) that exists above a sample of the liquid in a closed container. The vapour pressure of water is depressed by the degree of intermolecular interaction (intermolecular force) present in. What causes water's low vapor pressure? Nonvolatile liquids have low vapor pressures. As the temperature. What Causes Water's Low Vapor Pressure Quizlet.
From www.reicheltplumbing.com
What Causes Low Water Pressure Reichelt Plumbing What Causes Water's Low Vapor Pressure Quizlet The vapor pressure of a liquid varies with its temperature, as the following graph shows for water. Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; What causes water's low vapor pressure? When the vapor pressure equals the. The vapour pressure of water is depressed by the degree of intermolecular interaction (intermolecular. What Causes Water's Low Vapor Pressure Quizlet.
From www.slideserve.com
PPT Liquids, Solids, and Intermolecular Forces PowerPoint What Causes Water's Low Vapor Pressure Quizlet The phenomenon of vapor pressure. Study with quizlet and memorize flashcards containing terms like how does hydrogen bonding affect water molecules?, what causes water's. The vapor pressure of a liquid varies with its temperature, as the following graph shows for water. Nonvolatile liquids have low vapor pressures. Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily. What Causes Water's Low Vapor Pressure Quizlet.
From www.thespruce.com
Common Causes of Low Water Pressure What Causes Water's Low Vapor Pressure Quizlet Vapor pressure is defined as the partial pressure of a substance in the gas phase (vapor) that exists above a sample of the liquid in a closed container. As the temperature of a. Nonvolatile liquids have low vapor pressures. Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; The line on the. What Causes Water's Low Vapor Pressure Quizlet.
From www.slideserve.com
PPT VAPOR PRESSURE OF WATER PowerPoint Presentation, free download What Causes Water's Low Vapor Pressure Quizlet The phenomenon of vapor pressure. The vapour pressure of water is depressed by the degree of intermolecular interaction (intermolecular force) present in. As the temperature of a. Vapor pressure is defined as the partial pressure of a substance in the gas phase (vapor) that exists above a sample of the liquid in a closed container. When the vapor pressure equals. What Causes Water's Low Vapor Pressure Quizlet.
From www.scottsdaleplumbing.com
What Causes Low Water Pressure? What Causes Water's Low Vapor Pressure Quizlet Study with quizlet and memorize flashcards containing terms like how does hydrogen bonding affect water molecules?, what causes water's. Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; What causes water's low vapor pressure? Nonvolatile liquids have low vapor pressures. The vapor pressure of a liquid varies with its temperature, as the. What Causes Water's Low Vapor Pressure Quizlet.
From www.myxxgirl.com
Thermodynamics Predicting The Vapor Pressure Of Water My XXX Hot Girl What Causes Water's Low Vapor Pressure Quizlet The vapor pressure of a liquid varies with its temperature, as the following graph shows for water. Nonvolatile liquids have low vapor pressures. When the vapor pressure equals the. What causes water's low vapor pressure? Study with quizlet and memorize flashcards containing terms like how does hydrogen bonding affect water molecules?, what causes water's. As the temperature of a. Vapor. What Causes Water's Low Vapor Pressure Quizlet.
From slidetodoc.com
Chapter 15 water and aqueous systems Objectives Properties What Causes Water's Low Vapor Pressure Quizlet Study with quizlet and memorize flashcards containing terms like how does hydrogen bonding affect water molecules?, what causes water's. The vapour pressure of water is depressed by the degree of intermolecular interaction (intermolecular force) present in. The phenomenon of vapor pressure. The vapor pressure of a liquid varies with its temperature, as the following graph shows for water. What causes. What Causes Water's Low Vapor Pressure Quizlet.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID2437650 What Causes Water's Low Vapor Pressure Quizlet The phenomenon of vapor pressure. Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; The line on the graph shows the boiling temperature for water. Nonvolatile liquids have low vapor pressures. What causes water's low vapor pressure? Vapor pressure is defined as the partial pressure of a substance in the gas phase. What Causes Water's Low Vapor Pressure Quizlet.
From www.slideserve.com
PPT 6 Gases PowerPoint Presentation, free download ID352441 What Causes Water's Low Vapor Pressure Quizlet What causes water's low vapor pressure? Study with quizlet and memorize flashcards containing terms like how does hydrogen bonding affect water molecules?, what causes water's. The vapour pressure of water is depressed by the degree of intermolecular interaction (intermolecular force) present in. Nonvolatile liquids have low vapor pressures. The phenomenon of vapor pressure. When the vapor pressure equals the. The. What Causes Water's Low Vapor Pressure Quizlet.
From engineerexcel.com
Vapor Pressure of Water Explained EngineerExcel What Causes Water's Low Vapor Pressure Quizlet As the temperature of a. What causes water's low vapor pressure? Vapor pressure is defined as the partial pressure of a substance in the gas phase (vapor) that exists above a sample of the liquid in a closed container. The vapor pressure of a liquid varies with its temperature, as the following graph shows for water. Nonvolatile liquids have low. What Causes Water's Low Vapor Pressure Quizlet.
From saylordotorg.github.io
Vapor Pressure What Causes Water's Low Vapor Pressure Quizlet Nonvolatile liquids have low vapor pressures. Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; The vapor pressure of a liquid varies with its temperature, as the following graph shows for water. As the temperature of a. Study with quizlet and memorize flashcards containing terms like how does hydrogen bonding affect water. What Causes Water's Low Vapor Pressure Quizlet.
From www.numerade.com
SOLVED Water flows from a large tank as shown in the figure below What Causes Water's Low Vapor Pressure Quizlet What causes water's low vapor pressure? The line on the graph shows the boiling temperature for water. The phenomenon of vapor pressure. Study with quizlet and memorize flashcards containing terms like how does hydrogen bonding affect water molecules?, what causes water's. The vapor pressure of a liquid varies with its temperature, as the following graph shows for water. When the. What Causes Water's Low Vapor Pressure Quizlet.
From loezmirlu.blob.core.windows.net
Evaporation And Equilibrium Vapor Pressure at Cori Quiroz blog What Causes Water's Low Vapor Pressure Quizlet When the vapor pressure equals the. As the temperature of a. Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; The vapor pressure of a liquid varies with its temperature, as the following graph shows for water. The phenomenon of vapor pressure. Vapor pressure is defined as the partial pressure of a. What Causes Water's Low Vapor Pressure Quizlet.
From amariqoleon.blogspot.com
What is Vapor Pressure AmariqoLeon What Causes Water's Low Vapor Pressure Quizlet As the temperature of a. Nonvolatile liquids have low vapor pressures. The vapour pressure of water is depressed by the degree of intermolecular interaction (intermolecular force) present in. What causes water's low vapor pressure? The vapor pressure of a liquid varies with its temperature, as the following graph shows for water. Study with quizlet and memorize flashcards containing terms like. What Causes Water's Low Vapor Pressure Quizlet.
From www.slideserve.com
PPT VAPOR PRESSURE OF WATER PowerPoint Presentation, free download What Causes Water's Low Vapor Pressure Quizlet The vapour pressure of water is depressed by the degree of intermolecular interaction (intermolecular force) present in. The phenomenon of vapor pressure. What causes water's low vapor pressure? The vapor pressure of a liquid varies with its temperature, as the following graph shows for water. Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an. What Causes Water's Low Vapor Pressure Quizlet.
From slideplayer.com
Chapter 15 Review “Water and Aqueous Systems” ppt download What Causes Water's Low Vapor Pressure Quizlet The phenomenon of vapor pressure. Vapor pressure is defined as the partial pressure of a substance in the gas phase (vapor) that exists above a sample of the liquid in a closed container. What causes water's low vapor pressure? Study with quizlet and memorize flashcards containing terms like how does hydrogen bonding affect water molecules?, what causes water's. The vapor. What Causes Water's Low Vapor Pressure Quizlet.
From www.youtube.com
Evaporation, Vapor Pressure and Boiling YouTube What Causes Water's Low Vapor Pressure Quizlet Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; Study with quizlet and memorize flashcards containing terms like how does hydrogen bonding affect water molecules?, what causes water's. What causes water's low vapor pressure? As the temperature of a. The line on the graph shows the boiling temperature for water. The vapor. What Causes Water's Low Vapor Pressure Quizlet.
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It What Causes Water's Low Vapor Pressure Quizlet The phenomenon of vapor pressure. The vapor pressure of a liquid varies with its temperature, as the following graph shows for water. What causes water's low vapor pressure? Study with quizlet and memorize flashcards containing terms like how does hydrogen bonding affect water molecules?, what causes water's. Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily. What Causes Water's Low Vapor Pressure Quizlet.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID1025977 What Causes Water's Low Vapor Pressure Quizlet Volatile liquids are liquids with high vapor pressures, which tend to evaporate readily from an open container; The line on the graph shows the boiling temperature for water. As the temperature of a. Study with quizlet and memorize flashcards containing terms like how does hydrogen bonding affect water molecules?, what causes water's. Vapor pressure is defined as the partial pressure. What Causes Water's Low Vapor Pressure Quizlet.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID5080574 What Causes Water's Low Vapor Pressure Quizlet The vapour pressure of water is depressed by the degree of intermolecular interaction (intermolecular force) present in. What causes water's low vapor pressure? As the temperature of a. Nonvolatile liquids have low vapor pressures. When the vapor pressure equals the. The vapor pressure of a liquid varies with its temperature, as the following graph shows for water. The line on. What Causes Water's Low Vapor Pressure Quizlet.
From socratic.org
What is the relation between critical temperature and boiling point or What Causes Water's Low Vapor Pressure Quizlet Vapor pressure is defined as the partial pressure of a substance in the gas phase (vapor) that exists above a sample of the liquid in a closed container. The vapour pressure of water is depressed by the degree of intermolecular interaction (intermolecular force) present in. The phenomenon of vapor pressure. When the vapor pressure equals the. Study with quizlet and. What Causes Water's Low Vapor Pressure Quizlet.
From ar.inspiredpencil.com
Vapor Pressure Example What Causes Water's Low Vapor Pressure Quizlet The phenomenon of vapor pressure. Vapor pressure is defined as the partial pressure of a substance in the gas phase (vapor) that exists above a sample of the liquid in a closed container. As the temperature of a. Nonvolatile liquids have low vapor pressures. The line on the graph shows the boiling temperature for water. Study with quizlet and memorize. What Causes Water's Low Vapor Pressure Quizlet.
From www.numerade.com
SOLVED Question 8 3 pts According to the phase diagram of water as What Causes Water's Low Vapor Pressure Quizlet The line on the graph shows the boiling temperature for water. Vapor pressure is defined as the partial pressure of a substance in the gas phase (vapor) that exists above a sample of the liquid in a closed container. When the vapor pressure equals the. The vapour pressure of water is depressed by the degree of intermolecular interaction (intermolecular force). What Causes Water's Low Vapor Pressure Quizlet.