Visual Inspection Standard For Medical Devices . Visual inspection has been widely used for many years by the pharmaceutical, biologics and medical device industries after cleaning to release manufacturing equipment and devices. 1.4 this practice applies only to equipment or devices that have been justified through a quality risk management program to. Visual inspection has been widely used for many years by the pharmaceutical, biologics and medical device industries after cleaning to release. Part of the cleaning validation for the 21st century. Maceutical formulations or medical devices used for the qualiþcation of visual inspection. Standard practice for qualification of visual inspection of pharmaceutical manufacturing equipment and medical devices for residues 4.1. By andrew walsh, ralph basile, ovais mohammad, stéphane cousin, mariann neverovitch, and osamu shirokizawa. Some surrogate surfaces are actual samples of the medical devices themselves or smaller pieces of the.
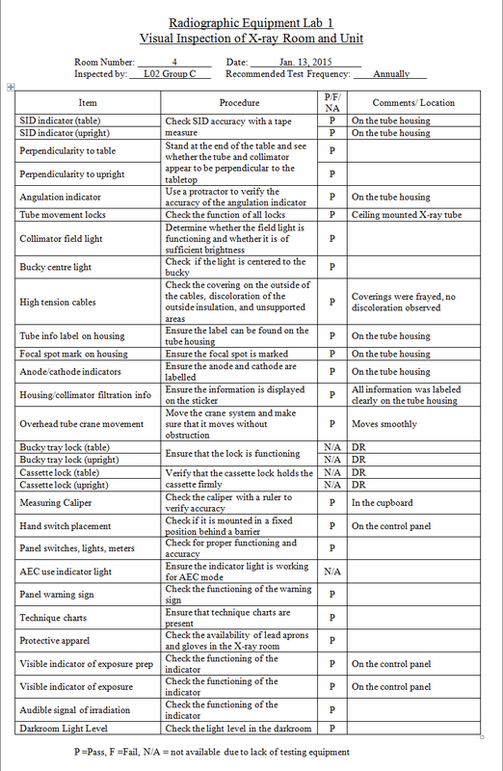
from qcinradiography.weebly.com
Maceutical formulations or medical devices used for the qualiþcation of visual inspection. Visual inspection has been widely used for many years by the pharmaceutical, biologics and medical device industries after cleaning to release manufacturing equipment and devices. Part of the cleaning validation for the 21st century. By andrew walsh, ralph basile, ovais mohammad, stéphane cousin, mariann neverovitch, and osamu shirokizawa. Visual inspection has been widely used for many years by the pharmaceutical, biologics and medical device industries after cleaning to release. Some surrogate surfaces are actual samples of the medical devices themselves or smaller pieces of the. Standard practice for qualification of visual inspection of pharmaceutical manufacturing equipment and medical devices for residues 4.1. 1.4 this practice applies only to equipment or devices that have been justified through a quality risk management program to.
Visual Inspection
Visual Inspection Standard For Medical Devices By andrew walsh, ralph basile, ovais mohammad, stéphane cousin, mariann neverovitch, and osamu shirokizawa. Maceutical formulations or medical devices used for the qualiþcation of visual inspection. By andrew walsh, ralph basile, ovais mohammad, stéphane cousin, mariann neverovitch, and osamu shirokizawa. Part of the cleaning validation for the 21st century. Visual inspection has been widely used for many years by the pharmaceutical, biologics and medical device industries after cleaning to release. Some surrogate surfaces are actual samples of the medical devices themselves or smaller pieces of the. 1.4 this practice applies only to equipment or devices that have been justified through a quality risk management program to. Visual inspection has been widely used for many years by the pharmaceutical, biologics and medical device industries after cleaning to release manufacturing equipment and devices. Standard practice for qualification of visual inspection of pharmaceutical manufacturing equipment and medical devices for residues 4.1.
From www.patlabelsonline.co.uk
Visual Inspection Labels for Portable Appliances Pat Labels Visual Inspection Standard For Medical Devices 1.4 this practice applies only to equipment or devices that have been justified through a quality risk management program to. Standard practice for qualification of visual inspection of pharmaceutical manufacturing equipment and medical devices for residues 4.1. Visual inspection has been widely used for many years by the pharmaceutical, biologics and medical device industries after cleaning to release manufacturing equipment. Visual Inspection Standard For Medical Devices.
From www.szmicmic.com
MIC1208 Automatic Visual Inspection Machine Visual Inspection Standard For Medical Devices Maceutical formulations or medical devices used for the qualiþcation of visual inspection. Visual inspection has been widely used for many years by the pharmaceutical, biologics and medical device industries after cleaning to release manufacturing equipment and devices. By andrew walsh, ralph basile, ovais mohammad, stéphane cousin, mariann neverovitch, and osamu shirokizawa. 1.4 this practice applies only to equipment or devices. Visual Inspection Standard For Medical Devices.
From www.knightoptical.com
Visual Inspection Knight Optical Visual Inspection Standard For Medical Devices Some surrogate surfaces are actual samples of the medical devices themselves or smaller pieces of the. Visual inspection has been widely used for many years by the pharmaceutical, biologics and medical device industries after cleaning to release. Maceutical formulations or medical devices used for the qualiþcation of visual inspection. Visual inspection has been widely used for many years by the. Visual Inspection Standard For Medical Devices.
From eureka.patsnap.com
Visual inspection system and method for glued products Eureka Patsnap Visual Inspection Standard For Medical Devices Some surrogate surfaces are actual samples of the medical devices themselves or smaller pieces of the. Visual inspection has been widely used for many years by the pharmaceutical, biologics and medical device industries after cleaning to release manufacturing equipment and devices. Standard practice for qualification of visual inspection of pharmaceutical manufacturing equipment and medical devices for residues 4.1. Part of. Visual Inspection Standard For Medical Devices.
From www.focusonndt.com
Visual Inspection NDT/NDE Rope Focus NDT Visual Inspection Standard For Medical Devices Part of the cleaning validation for the 21st century. Maceutical formulations or medical devices used for the qualiþcation of visual inspection. Visual inspection has been widely used for many years by the pharmaceutical, biologics and medical device industries after cleaning to release manufacturing equipment and devices. Some surrogate surfaces are actual samples of the medical devices themselves or smaller pieces. Visual Inspection Standard For Medical Devices.
From eureka.patsnap.com
Aluminum flatness visual inspection system Eureka Patsnap Visual Inspection Standard For Medical Devices Some surrogate surfaces are actual samples of the medical devices themselves or smaller pieces of the. Standard practice for qualification of visual inspection of pharmaceutical manufacturing equipment and medical devices for residues 4.1. Visual inspection has been widely used for many years by the pharmaceutical, biologics and medical device industries after cleaning to release. 1.4 this practice applies only to. Visual Inspection Standard For Medical Devices.
From www.inneseyeclinic.com
Here’s What Goes on During a Standard Eye Exam Laurier Optical Visual Inspection Standard For Medical Devices Maceutical formulations or medical devices used for the qualiþcation of visual inspection. Some surrogate surfaces are actual samples of the medical devices themselves or smaller pieces of the. By andrew walsh, ralph basile, ovais mohammad, stéphane cousin, mariann neverovitch, and osamu shirokizawa. Standard practice for qualification of visual inspection of pharmaceutical manufacturing equipment and medical devices for residues 4.1. 1.4. Visual Inspection Standard For Medical Devices.
From qcinradiography.weebly.com
Visual Inspection Visual Inspection Standard For Medical Devices Maceutical formulations or medical devices used for the qualiþcation of visual inspection. By andrew walsh, ralph basile, ovais mohammad, stéphane cousin, mariann neverovitch, and osamu shirokizawa. Part of the cleaning validation for the 21st century. Visual inspection has been widely used for many years by the pharmaceutical, biologics and medical device industries after cleaning to release. Standard practice for qualification. Visual Inspection Standard For Medical Devices.
From datamyte.com
Visual Inspection for Quality Control Checklist Full Guide DataMyte Visual Inspection Standard For Medical Devices Standard practice for qualification of visual inspection of pharmaceutical manufacturing equipment and medical devices for residues 4.1. Some surrogate surfaces are actual samples of the medical devices themselves or smaller pieces of the. Visual inspection has been widely used for many years by the pharmaceutical, biologics and medical device industries after cleaning to release manufacturing equipment and devices. Maceutical formulations. Visual Inspection Standard For Medical Devices.
From www.fogwing.io
New Visual Inspection Techniques Fogwing.io Visual Inspection Standard For Medical Devices Standard practice for qualification of visual inspection of pharmaceutical manufacturing equipment and medical devices for residues 4.1. Part of the cleaning validation for the 21st century. Visual inspection has been widely used for many years by the pharmaceutical, biologics and medical device industries after cleaning to release. Visual inspection has been widely used for many years by the pharmaceutical, biologics. Visual Inspection Standard For Medical Devices.
From www.researchgate.net
Visual Inspection Checklist (new version) Download Scientific Diagram Visual Inspection Standard For Medical Devices Standard practice for qualification of visual inspection of pharmaceutical manufacturing equipment and medical devices for residues 4.1. Visual inspection has been widely used for many years by the pharmaceutical, biologics and medical device industries after cleaning to release. Visual inspection has been widely used for many years by the pharmaceutical, biologics and medical device industries after cleaning to release manufacturing. Visual Inspection Standard For Medical Devices.
From www.skillcatapp.com
Visual Inspection Visual Inspection Standard For Medical Devices By andrew walsh, ralph basile, ovais mohammad, stéphane cousin, mariann neverovitch, and osamu shirokizawa. Visual inspection has been widely used for many years by the pharmaceutical, biologics and medical device industries after cleaning to release manufacturing equipment and devices. 1.4 this practice applies only to equipment or devices that have been justified through a quality risk management program to. Part. Visual Inspection Standard For Medical Devices.
From www.ubi-inc.net
Visual Inspection Module UBI, Inc. Visual Inspection Standard For Medical Devices Part of the cleaning validation for the 21st century. Maceutical formulations or medical devices used for the qualiþcation of visual inspection. Some surrogate surfaces are actual samples of the medical devices themselves or smaller pieces of the. By andrew walsh, ralph basile, ovais mohammad, stéphane cousin, mariann neverovitch, and osamu shirokizawa. Standard practice for qualification of visual inspection of pharmaceutical. Visual Inspection Standard For Medical Devices.
From www.labelbar.co.uk
4th Edition Visual Inspection 4 Colours Cable Wrap Labels Visual Inspection Standard For Medical Devices Standard practice for qualification of visual inspection of pharmaceutical manufacturing equipment and medical devices for residues 4.1. 1.4 this practice applies only to equipment or devices that have been justified through a quality risk management program to. By andrew walsh, ralph basile, ovais mohammad, stéphane cousin, mariann neverovitch, and osamu shirokizawa. Part of the cleaning validation for the 21st century.. Visual Inspection Standard For Medical Devices.
From www.labelbar.co.uk
Custom 4th/5th Edition PAT Testing Visual Inspection Labels Visual Inspection Standard For Medical Devices 1.4 this practice applies only to equipment or devices that have been justified through a quality risk management program to. Maceutical formulations or medical devices used for the qualiþcation of visual inspection. Visual inspection has been widely used for many years by the pharmaceutical, biologics and medical device industries after cleaning to release manufacturing equipment and devices. Some surrogate surfaces. Visual Inspection Standard For Medical Devices.
From www.ashvision.com
Medical Device Inspection ASH Visual Inspection Standard For Medical Devices Visual inspection has been widely used for many years by the pharmaceutical, biologics and medical device industries after cleaning to release. Maceutical formulations or medical devices used for the qualiþcation of visual inspection. 1.4 this practice applies only to equipment or devices that have been justified through a quality risk management program to. Some surrogate surfaces are actual samples of. Visual Inspection Standard For Medical Devices.
From www.indiamart.com
Remote Visual Inspection Services, Forensic Technological Innovation Visual Inspection Standard For Medical Devices By andrew walsh, ralph basile, ovais mohammad, stéphane cousin, mariann neverovitch, and osamu shirokizawa. Part of the cleaning validation for the 21st century. 1.4 this practice applies only to equipment or devices that have been justified through a quality risk management program to. Visual inspection has been widely used for many years by the pharmaceutical, biologics and medical device industries. Visual Inspection Standard For Medical Devices.
From www.slideserve.com
PPT Enhancing Workplace Safety FTI Incorporation's Certified Visual Inspection Standard For Medical Devices Maceutical formulations or medical devices used for the qualiþcation of visual inspection. Standard practice for qualification of visual inspection of pharmaceutical manufacturing equipment and medical devices for residues 4.1. Some surrogate surfaces are actual samples of the medical devices themselves or smaller pieces of the. Visual inspection has been widely used for many years by the pharmaceutical, biologics and medical. Visual Inspection Standard For Medical Devices.
From www-engineering.blogspot.com
Engineering Visual Inspection Standard For Medical Devices Visual inspection has been widely used for many years by the pharmaceutical, biologics and medical device industries after cleaning to release. 1.4 this practice applies only to equipment or devices that have been justified through a quality risk management program to. Maceutical formulations or medical devices used for the qualiþcation of visual inspection. Part of the cleaning validation for the. Visual Inspection Standard For Medical Devices.
From www.apizee.com
A Simple Guide to Remote Visual Inspection Apizee Visual Inspection Standard For Medical Devices Part of the cleaning validation for the 21st century. Maceutical formulations or medical devices used for the qualiþcation of visual inspection. Visual inspection has been widely used for many years by the pharmaceutical, biologics and medical device industries after cleaning to release manufacturing equipment and devices. Standard practice for qualification of visual inspection of pharmaceutical manufacturing equipment and medical devices. Visual Inspection Standard For Medical Devices.
From www.nichiwak.co.jp
Sorting into Trays, Die Visual Inspection (Inspector/Machine) and Trays Visual Inspection Standard For Medical Devices Part of the cleaning validation for the 21st century. 1.4 this practice applies only to equipment or devices that have been justified through a quality risk management program to. By andrew walsh, ralph basile, ovais mohammad, stéphane cousin, mariann neverovitch, and osamu shirokizawa. Some surrogate surfaces are actual samples of the medical devices themselves or smaller pieces of the. Visual. Visual Inspection Standard For Medical Devices.
From www.sipotek.net
Automated Optical Vision Inspection Machine Malaysia Sipotek Visual Visual Inspection Standard For Medical Devices Visual inspection has been widely used for many years by the pharmaceutical, biologics and medical device industries after cleaning to release manufacturing equipment and devices. Maceutical formulations or medical devices used for the qualiþcation of visual inspection. Visual inspection has been widely used for many years by the pharmaceutical, biologics and medical device industries after cleaning to release. Part of. Visual Inspection Standard For Medical Devices.
From www.awsbiopharma.com
Inspection Systems AWS BioPharma Technologies Visual Inspection Standard For Medical Devices 1.4 this practice applies only to equipment or devices that have been justified through a quality risk management program to. By andrew walsh, ralph basile, ovais mohammad, stéphane cousin, mariann neverovitch, and osamu shirokizawa. Standard practice for qualification of visual inspection of pharmaceutical manufacturing equipment and medical devices for residues 4.1. Visual inspection has been widely used for many years. Visual Inspection Standard For Medical Devices.
From www.opto-system.co.jp
Visual Inspection Machine VVF4000 Opto System Co., Ltd. Visual Inspection Standard For Medical Devices Visual inspection has been widely used for many years by the pharmaceutical, biologics and medical device industries after cleaning to release manufacturing equipment and devices. 1.4 this practice applies only to equipment or devices that have been justified through a quality risk management program to. Some surrogate surfaces are actual samples of the medical devices themselves or smaller pieces of. Visual Inspection Standard For Medical Devices.
From www.vetter-pharma.com
Locations Manual Visual Inspection (VSP2) Vetter Visual Inspection Standard For Medical Devices Part of the cleaning validation for the 21st century. Visual inspection has been widely used for many years by the pharmaceutical, biologics and medical device industries after cleaning to release. Visual inspection has been widely used for many years by the pharmaceutical, biologics and medical device industries after cleaning to release manufacturing equipment and devices. Maceutical formulations or medical devices. Visual Inspection Standard For Medical Devices.
From metrology.news
Automated Optical Inspection Versus Traditional Manual Inspection Visual Inspection Standard For Medical Devices 1.4 this practice applies only to equipment or devices that have been justified through a quality risk management program to. Some surrogate surfaces are actual samples of the medical devices themselves or smaller pieces of the. Visual inspection has been widely used for many years by the pharmaceutical, biologics and medical device industries after cleaning to release manufacturing equipment and. Visual Inspection Standard For Medical Devices.
From www.ombrulla.com
AI Visual Inspection AI Defect Detection Computer Vision Visual Inspection Standard For Medical Devices 1.4 this practice applies only to equipment or devices that have been justified through a quality risk management program to. Part of the cleaning validation for the 21st century. By andrew walsh, ralph basile, ovais mohammad, stéphane cousin, mariann neverovitch, and osamu shirokizawa. Maceutical formulations or medical devices used for the qualiþcation of visual inspection. Standard practice for qualification of. Visual Inspection Standard For Medical Devices.
From ftiincorporation.hashnode.dev
Visual Inspection Standards and Qualification Visual Inspection Standard For Medical Devices Visual inspection has been widely used for many years by the pharmaceutical, biologics and medical device industries after cleaning to release. By andrew walsh, ralph basile, ovais mohammad, stéphane cousin, mariann neverovitch, and osamu shirokizawa. Some surrogate surfaces are actual samples of the medical devices themselves or smaller pieces of the. Part of the cleaning validation for the 21st century.. Visual Inspection Standard For Medical Devices.
From www.dochub.com
Visual inspection checklist template Fill out & sign online DocHub Visual Inspection Standard For Medical Devices Visual inspection has been widely used for many years by the pharmaceutical, biologics and medical device industries after cleaning to release manufacturing equipment and devices. 1.4 this practice applies only to equipment or devices that have been justified through a quality risk management program to. Some surrogate surfaces are actual samples of the medical devices themselves or smaller pieces of. Visual Inspection Standard For Medical Devices.
From eureka.patsnap.com
Automatic equipment for multidirection visual inspection Eureka Visual Inspection Standard For Medical Devices By andrew walsh, ralph basile, ovais mohammad, stéphane cousin, mariann neverovitch, and osamu shirokizawa. Standard practice for qualification of visual inspection of pharmaceutical manufacturing equipment and medical devices for residues 4.1. Visual inspection has been widely used for many years by the pharmaceutical, biologics and medical device industries after cleaning to release manufacturing equipment and devices. Some surrogate surfaces are. Visual Inspection Standard For Medical Devices.
From www.gdcompt.com
Visual inspection equipment Visual Inspection Standard For Medical Devices Visual inspection has been widely used for many years by the pharmaceutical, biologics and medical device industries after cleaning to release. 1.4 this practice applies only to equipment or devices that have been justified through a quality risk management program to. Standard practice for qualification of visual inspection of pharmaceutical manufacturing equipment and medical devices for residues 4.1. Visual inspection. Visual Inspection Standard For Medical Devices.
From www.mitsubishielectric.com
Visual Inspection Software MELSOFT VIXIO Products MITSUBISHI Visual Inspection Standard For Medical Devices 1.4 this practice applies only to equipment or devices that have been justified through a quality risk management program to. Visual inspection has been widely used for many years by the pharmaceutical, biologics and medical device industries after cleaning to release. Maceutical formulations or medical devices used for the qualiþcation of visual inspection. Some surrogate surfaces are actual samples of. Visual Inspection Standard For Medical Devices.
From www.opto-system.co.jp
Visual Inspection Sorter VVSP4000 Opto System Co., Ltd. Visual Inspection Standard For Medical Devices 1.4 this practice applies only to equipment or devices that have been justified through a quality risk management program to. Maceutical formulations or medical devices used for the qualiþcation of visual inspection. Visual inspection has been widely used for many years by the pharmaceutical, biologics and medical device industries after cleaning to release. Some surrogate surfaces are actual samples of. Visual Inspection Standard For Medical Devices.
From formspal.com
Visual Inspection Form ≡ Fill Out Printable PDF Forms Online Visual Inspection Standard For Medical Devices Part of the cleaning validation for the 21st century. Visual inspection has been widely used for many years by the pharmaceutical, biologics and medical device industries after cleaning to release. Maceutical formulations or medical devices used for the qualiþcation of visual inspection. 1.4 this practice applies only to equipment or devices that have been justified through a quality risk management. Visual Inspection Standard For Medical Devices.
From www.linkedin.com
Visual Inspection Solutions for FMCG Products Enhancing Quality Control Visual Inspection Standard For Medical Devices Visual inspection has been widely used for many years by the pharmaceutical, biologics and medical device industries after cleaning to release manufacturing equipment and devices. Standard practice for qualification of visual inspection of pharmaceutical manufacturing equipment and medical devices for residues 4.1. 1.4 this practice applies only to equipment or devices that have been justified through a quality risk management. Visual Inspection Standard For Medical Devices.