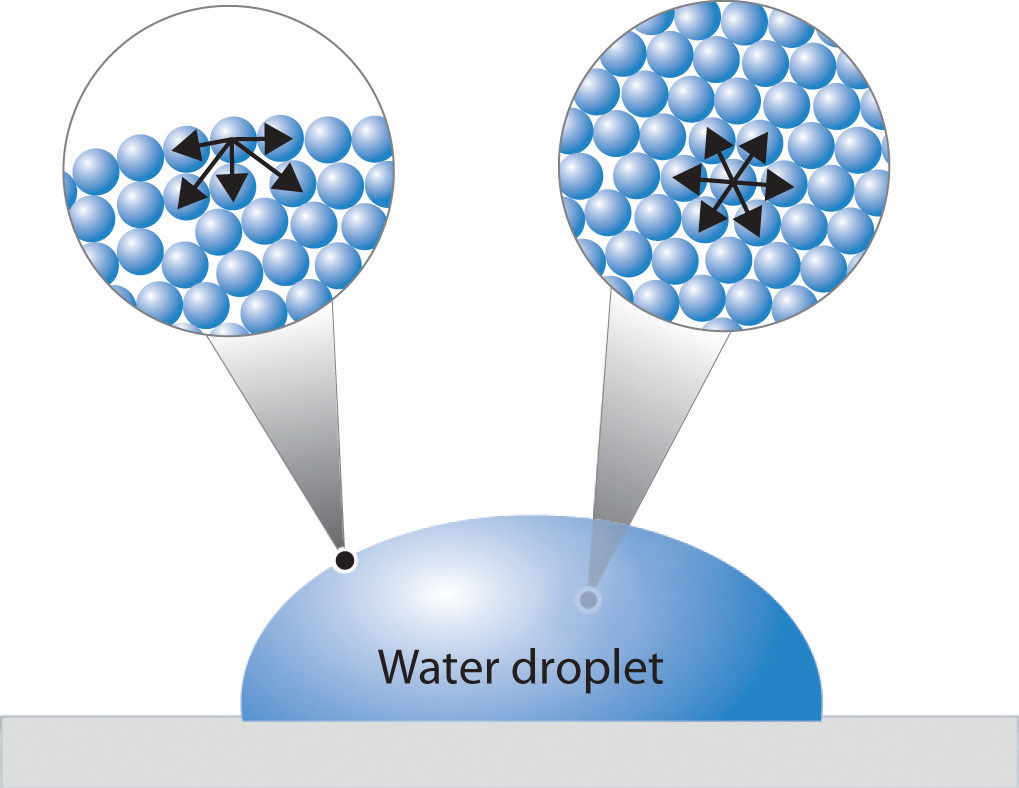
Surface Tension Intermolecular Forces . surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. in this article, you will learn about the effects of intermolecular forces (imfs). the surface tension of a liquid is a measure of the elastic force in the liquid's surface. surface tension | states of matter and intermolecular forces. surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly. You will understand how intermolecular forces influence the physical. The surface tension of a liquid results from an.
from chem.libretexts.org
in this article, you will learn about the effects of intermolecular forces (imfs). surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. You will understand how intermolecular forces influence the physical. surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly. surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. The surface tension of a liquid results from an. surface tension | states of matter and intermolecular forces. the surface tension of a liquid is a measure of the elastic force in the liquid's surface.
11.4 Intermolecular Forces in Action Surface Tension, Viscosity, and Capillary Action
Surface Tension Intermolecular Forces the surface tension of a liquid is a measure of the elastic force in the liquid's surface. The surface tension of a liquid results from an. You will understand how intermolecular forces influence the physical. surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. the surface tension of a liquid is a measure of the elastic force in the liquid's surface. surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly. surface tension | states of matter and intermolecular forces. surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. in this article, you will learn about the effects of intermolecular forces (imfs).
From studylib.net
INTERMOLECULAR FORCES Surface Tension Intermolecular Forces surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. surface tension | states of matter and intermolecular forces. in this article,. Surface Tension Intermolecular Forces.
From www.ilectureonline.com
Surface Tension Intermolecular Forces surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly. the surface tension of a liquid is a measure of the elastic force in the liquid's surface. in this article, you will learn about the effects of intermolecular forces (imfs). surface tension, the amount of. Surface Tension Intermolecular Forces.
From slideplayer.com
Intermolecular forces ppt download Surface Tension Intermolecular Forces The surface tension of a liquid results from an. surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. the surface tension of a liquid is a measure of the elastic force in the liquid's surface. in this article, you will learn about the effects of. Surface Tension Intermolecular Forces.
From www.researchgate.net
2. Intermolecular forces causing the surface tension phenomena (A) and... Download Scientific Surface Tension Intermolecular Forces surface tension | states of matter and intermolecular forces. You will understand how intermolecular forces influence the physical. the surface tension of a liquid is a measure of the elastic force in the liquid's surface. surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. . Surface Tension Intermolecular Forces.
From slideplayer.com
Chapter 11 Liquids, solids, and intermolecular forces ppt download Surface Tension Intermolecular Forces You will understand how intermolecular forces influence the physical. surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. the surface tension of a liquid is a measure of the elastic force in the liquid's surface. surface tension is measured as the energy required. Surface Tension Intermolecular Forces.
From www.youtube.com
Surface tension and capillary action Intermolecular Forces meriSTEM YouTube Surface Tension Intermolecular Forces surface tension | states of matter and intermolecular forces. surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. The surface tension of a liquid results from an. You will understand how intermolecular forces influence the physical. surface tension is measured as the energy. Surface Tension Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular forces Generalizing properties PowerPoint Presentation ID6049698 Surface Tension Intermolecular Forces surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly. the surface tension of a liquid is a measure of the elastic force in the liquid's surface. surface tension is measured as the energy required to increase the surface area of a liquid by a unit. Surface Tension Intermolecular Forces.
From slideplayer.com
Intermolecular forces ppt download Surface Tension Intermolecular Forces in this article, you will learn about the effects of intermolecular forces (imfs). surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. The surface tension of a liquid results from an. surface tension, the amount of energy required to increase the surface area of a. Surface Tension Intermolecular Forces.
From www.youtube.com
Introduction of Surface tension/intermolecular force/range of molecular force/sphere of Surface Tension Intermolecular Forces the surface tension of a liquid is a measure of the elastic force in the liquid's surface. surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly. surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property. Surface Tension Intermolecular Forces.
From www.youtube.com
CHEMISTRY 101 Effects of intermolecular forces on surface tension, viscosity, and capillary Surface Tension Intermolecular Forces surface tension | states of matter and intermolecular forces. You will understand how intermolecular forces influence the physical. surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly. the surface tension of a liquid is a measure of the elastic force in the liquid's surface. . Surface Tension Intermolecular Forces.
From www.youtube.com
11.4 Intermolecular Forces in Action Surface Tension, Viscosity, & Capillary Action YouTube Surface Tension Intermolecular Forces in this article, you will learn about the effects of intermolecular forces (imfs). the surface tension of a liquid is a measure of the elastic force in the liquid's surface. surface tension | states of matter and intermolecular forces. surface tension is the energy required to increase the surface area of a liquid by a unit. Surface Tension Intermolecular Forces.
From www.youtube.com
Surface tension States of matter and intermolecular forces Chemistry Khan Academy YouTube Surface Tension Intermolecular Forces The surface tension of a liquid results from an. surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly. in this article, you will learn about the effects of intermolecular forces (imfs). surface tension | states of matter and intermolecular forces. surface tension, the amount. Surface Tension Intermolecular Forces.
From www.youtube.com
Surface tension Intermolecular forces YouTube Surface Tension Intermolecular Forces You will understand how intermolecular forces influence the physical. The surface tension of a liquid results from an. surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. in this article, you will learn about the effects of intermolecular forces (imfs). surface tension is. Surface Tension Intermolecular Forces.
From www.youtube.com
12.3 Intermolecular Forces in Action Surface Tension & Viscosity YouTube Surface Tension Intermolecular Forces surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly. the surface tension of a liquid is a measure of the elastic force in the liquid's surface. surface tension | states of matter and intermolecular forces. You will understand how intermolecular forces influence the physical. . Surface Tension Intermolecular Forces.
From www.youtube.com
11.4 Intermolecular Forces in Action Surface Tension, Viscosity, & Capillary Action YouTube Surface Tension Intermolecular Forces surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly. The surface tension of a liquid results from an. in this article, you will learn about the effects of intermolecular forces (imfs). surface tension | states of matter and intermolecular forces. surface tension is measured. Surface Tension Intermolecular Forces.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance Surface Tension Intermolecular Forces the surface tension of a liquid is a measure of the elastic force in the liquid's surface. surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property. Surface Tension Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces, Liquids and Solids PowerPoint Presentation ID376421 Surface Tension Intermolecular Forces surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly. surface tension | states of matter and intermolecular forces. in this article, you will. Surface Tension Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces of Attraction PowerPoint Presentation, free download ID260295 Surface Tension Intermolecular Forces surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. surface tension | states of matter and intermolecular forces. surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. in this article,. Surface Tension Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID2081010 Surface Tension Intermolecular Forces surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly. the surface tension of a liquid is a measure of the elastic force in the liquid's surface. surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property. Surface Tension Intermolecular Forces.
From www.slideserve.com
PPT Implication of Intermolecular Forces PowerPoint Presentation, free download ID3521234 Surface Tension Intermolecular Forces surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. the surface tension of a liquid is a measure of the elastic force in the liquid's surface. surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property. Surface Tension Intermolecular Forces.
From www.studypool.com
SOLUTION Intermolecular forces and surface tension docx Studypool Surface Tension Intermolecular Forces surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. surface tension | states of matter and intermolecular forces. in this article, you will learn about the effects of intermolecular forces (imfs). surface tension is the energy required to increase the surface area of a. Surface Tension Intermolecular Forces.
From www.youtube.com
surface tensionintermolecular forcessurface energyproperties of matter11 Physicssky physics Surface Tension Intermolecular Forces surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly. in this article, you will learn about the effects of intermolecular forces (imfs). surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces.. Surface Tension Intermolecular Forces.
From chem.libretexts.org
11.4 Intermolecular Forces in Action Surface Tension, Viscosity, and Capillary Action Surface Tension Intermolecular Forces surface tension | states of matter and intermolecular forces. surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. the surface tension. Surface Tension Intermolecular Forces.
From www.youtube.com
Intermolecular Forces Trends Melting & Boiling Point, Viscosity, Surface Tension, Vapor Surface Tension Intermolecular Forces in this article, you will learn about the effects of intermolecular forces (imfs). surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. the surface tension of a liquid is a measure of the elastic force in the liquid's surface. You will understand how. Surface Tension Intermolecular Forces.
From www.pearson.com
Intermolecular Forces and Physical Properties Example 1 Channels for Pearson+ Surface Tension Intermolecular Forces The surface tension of a liquid results from an. You will understand how intermolecular forces influence the physical. in this article, you will learn about the effects of intermolecular forces (imfs). surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly. surface tension | states of. Surface Tension Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID5042599 Surface Tension Intermolecular Forces The surface tension of a liquid results from an. surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. the surface tension of. Surface Tension Intermolecular Forces.
From www.slideserve.com
PPT Implication of Intermolecular Forces PowerPoint Presentation, free download ID3521234 Surface Tension Intermolecular Forces surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly. in this article, you will learn about the effects of intermolecular forces (imfs).. Surface Tension Intermolecular Forces.
From www.studocu.com
4.7 Intermolecular Forces INTERMOLECULAR FORCES LAB STATION 1 SURFACE TENSION LEARNING TARGET Surface Tension Intermolecular Forces You will understand how intermolecular forces influence the physical. in this article, you will learn about the effects of intermolecular forces (imfs). surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. surface tension | states of matter and intermolecular forces. surface tension. Surface Tension Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces and Liquids and Solids PowerPoint Presentation ID3374064 Surface Tension Intermolecular Forces You will understand how intermolecular forces influence the physical. in this article, you will learn about the effects of intermolecular forces (imfs). surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly. The surface tension of a liquid results from an. the surface tension of a. Surface Tension Intermolecular Forces.
From 88guru.com
Viscosity And Surface Tension Definition, Applications and FAQ's 88Guru Surface Tension Intermolecular Forces surface tension | states of matter and intermolecular forces. surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. The surface tension of a liquid results from an. surface tension is the energy required to increase the surface area of a liquid by a. Surface Tension Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces and Liquids and Solids PowerPoint Presentation ID1770193 Surface Tension Intermolecular Forces surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly. surface tension | states of matter and intermolecular forces. You will understand how intermolecular forces influence the physical. the surface tension of a liquid is a measure of the elastic force in the liquid's surface. . Surface Tension Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID3374111 Surface Tension Intermolecular Forces in this article, you will learn about the effects of intermolecular forces (imfs). surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. the surface tension of a liquid is a measure of the elastic force in the liquid's surface. You will understand how. Surface Tension Intermolecular Forces.
From www.chemedx.org
Measuring Surface Tension to Investigate Intermolecular Forces Chemical Education Xchange Surface Tension Intermolecular Forces surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. You will understand how intermolecular forces influence the physical. surface tension | states of matter and intermolecular forces. The surface tension of a liquid results from an. in this article, you will learn about. Surface Tension Intermolecular Forces.
From www.youtube.com
Intermolecular Forces, Surface Tension, & Evaporation YouTube Surface Tension Intermolecular Forces surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. the surface tension of a liquid is a measure of the elastic force in the liquid's surface. surface tension | states of matter and intermolecular forces. in this article, you will learn about. Surface Tension Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID2081010 Surface Tension Intermolecular Forces You will understand how intermolecular forces influence the physical. surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. The surface tension of a liquid results from an. surface tension is measured as the energy required to increase the surface area of a liquid by. Surface Tension Intermolecular Forces.