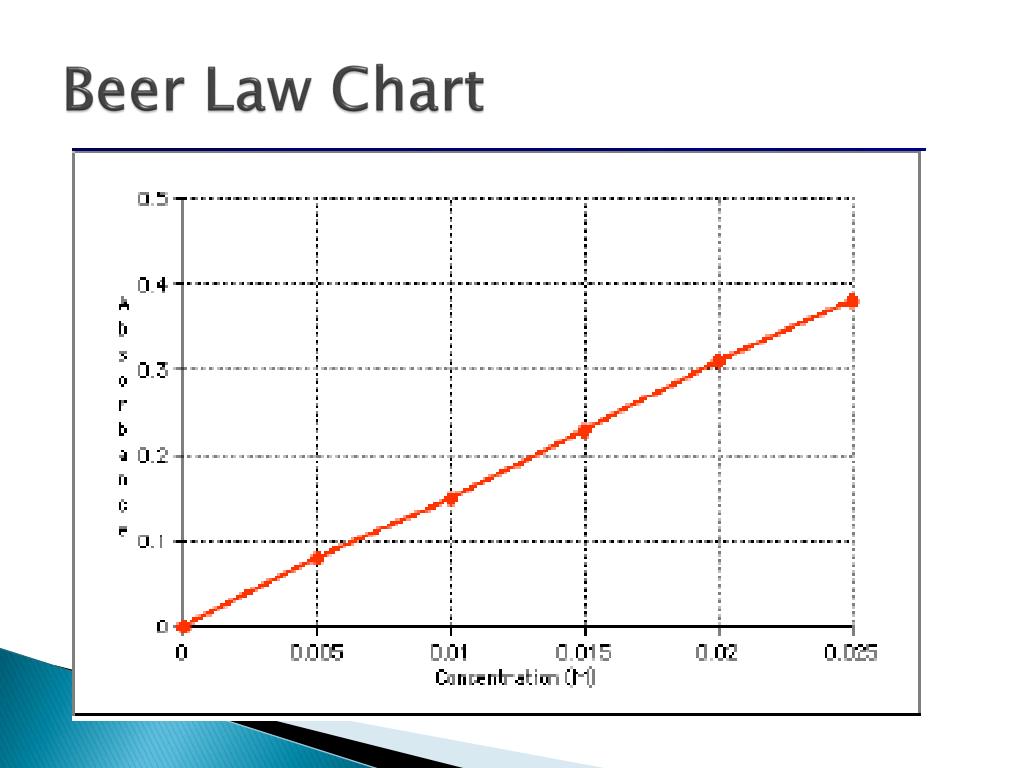
Beer's Law Temperature . Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In other words, a solution. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. The premise is that a light beam becomes weaker as. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration.
from www.slideserve.com
Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The premise is that a light beam becomes weaker as. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. In other words, a solution. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration.
PPT chapter 2 PowerPoint Presentation, free download ID540228
Beer's Law Temperature Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In other words, a solution. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. The premise is that a light beam becomes weaker as.
From phoenixlandingbar.com
What Temperature Do You Serve Beer In A Keg? Beer's Law Temperature Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In other words, a solution. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Beer's law states that a chemical solution's concentration is directly. Beer's Law Temperature.
From rrennenextanner.blogspot.com
Beer Lambert Law Ppt rrennenexTanner Beer's Law Temperature Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In other words, a solution. The premise is that a light beam becomes weaker as. In spectroscopy, beer’s law states. Beer's Law Temperature.
From www.scienceabc.com
Beers Law Definition, History, Equation, Formula And Example Beer's Law Temperature In other words, a solution. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. The premise is that a light beam becomes weaker as. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Since the concentration, path length. Beer's Law Temperature.
From www.youtube.com
Beer Lambert law derivation and usage YouTube Beer's Law Temperature In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. The premise is that a light beam becomes weaker as. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as.. Beer's Law Temperature.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law Temperature Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. The premise is that a light beam becomes weaker as.. Beer's Law Temperature.
From lukewarmtakes.net
Spectrophotometry and Beer's Law Lukewarm Takes Beer's Law Temperature Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. In other words, a solution. The premise is that a light beam becomes weaker as. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. In spectroscopy, beer’s law states that the absorption of light. Beer's Law Temperature.
From ecency.com
Spectrophotometry Simplified The BeerLambert Law in Spectrophotom... Beer's Law Temperature Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. In other words, a solution. Beer's law states that a chemical solution's concentration is directly. Beer's Law Temperature.
From www.brewersjournal.info
Science Warm maturation and cold stabilisation The Brewers Journal Beer's Law Temperature In other words, a solution. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance,. Beer's Law Temperature.
From calculatorghw.blogspot.com
Beer Lambert Law Calculator CALCULATOR GHW Beer's Law Temperature Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. The premise is that a light beam becomes weaker as. In other words, a solution. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. In spectroscopy, beer’s law states that the absorption of light. Beer's Law Temperature.
From chemistrytalk.org
BeerLambert Law ChemTalk Beer's Law Temperature In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. In other words, a solution. The premise is that a light beam becomes weaker as.. Beer's Law Temperature.
From www.researchgate.net
1 a Schematic representation for BeerLambert law for the measurement Beer's Law Temperature Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In other words, a solution. In spectroscopy, beer’s law states that the absorption of light. Beer's Law Temperature.
From commonsenseevaluation.com
The Perfect Temperatures For Beverages Common Sense Evaluation Beer's Law Temperature In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. In other words, a solution. The premise is that a light beam becomes weaker as. Since the concentration, path length. Beer's Law Temperature.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Beer's Law Temperature The premise is that a light beam becomes weaker as. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Beer's law states that a. Beer's Law Temperature.
From www.slideserve.com
PPT Beer’s Law & Colorimetry PowerPoint Presentation, free download Beer's Law Temperature In other words, a solution. The premise is that a light beam becomes weaker as. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In spectroscopy, beer’s law states. Beer's Law Temperature.
From beertoday.co.uk
Getting a handle on the perfect beer temperature Beer Today Beer's Law Temperature The premise is that a light beam becomes weaker as. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In other words, a solution.. Beer's Law Temperature.
From www.oculyze.net
Beer Fermentation Temperature Chart A Complete Guide Beer's Law Temperature In other words, a solution. The premise is that a light beam becomes weaker as. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as.. Beer's Law Temperature.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law Temperature The premise is that a light beam becomes weaker as. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Since the concentration, path length. Beer's Law Temperature.
From chem.libretexts.org
10.2 Spectroscopy Based on Absorption Chemistry LibreTexts Beer's Law Temperature Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. In other words, a solution. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer's Law Temperature.
From homebarkit.com
What Is The Ideal Temperature For Drinks? Home Bar Kit Beer's Law Temperature Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. In other words, a solution. The premise is that a light beam becomes weaker as. Beer lamberts law states a. Beer's Law Temperature.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Beer's Law Temperature In other words, a solution. The premise is that a light beam becomes weaker as. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of. Beer's Law Temperature.
From www.craftbeerjoe.com
Craft Beer Temperature Guide Craft Beer Joe Beer's Law Temperature In other words, a solution. The premise is that a light beam becomes weaker as. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Since the concentration, path length and molar absorptivity are all. Beer's Law Temperature.
From www.slideserve.com
PPT Atmospheric Transmission PowerPoint Presentation, free download Beer's Law Temperature The premise is that a light beam becomes weaker as. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as.. Beer's Law Temperature.
From www.slideserve.com
PPT chapter 2 PowerPoint Presentation, free download ID540228 Beer's Law Temperature In other words, a solution. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer's Law Temperature.
From www.oculyze.net
Beer Serving Temperature Chart Beer's Law Temperature Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In other words, a solution. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. The premise is that a. Beer's Law Temperature.
From www.oculyze.net
Beer Serving Temperature Chart Beer's Law Temperature In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In other words, a solution. The premise is that a. Beer's Law Temperature.
From www.youtube.com
Beer's Law Overview YouTube Beer's Law Temperature In other words, a solution. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of. Beer's Law Temperature.
From www.slideserve.com
PPT Determining the Concentration of a Solution Beer’s Law Beer's Law Temperature Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. The premise is that a light beam becomes weaker as. In other words, a solution. In spectroscopy, beer’s law states that the absorption of light. Beer's Law Temperature.
From www.researchgate.net
Validation for the use of Beer's law for dimeric species at specific Beer's Law Temperature In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. In other words, a solution. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Since the concentration, path length and molar absorptivity are all. Beer's Law Temperature.
From www.winemag.com
Does Beer Temperature Matter? How to Serve IPAs, Cask Ales and More Beer's Law Temperature Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. In other words, a solution. The premise is that a light beam becomes weaker as. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration.. Beer's Law Temperature.
From www.youtube.com
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry Beer's Law Temperature Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. In other words, a solution. In spectroscopy, beer’s law states that the absorption of light by a sample is directly. Beer's Law Temperature.
From www.wineenthusiast.com
Does Beer Temperature Matter? How to Serve IPAs, Cask Ales and More Beer's Law Temperature In other words, a solution. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Beer's law states that a chemical solution's concentration is directly. Beer's Law Temperature.
From sciencenotes.org
Beer's Law Equation and Example Beer's Law Temperature Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. The premise is that a light beam becomes weaker as. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In spectroscopy, beer’s law states. Beer's Law Temperature.
From www.slideserve.com
PPT Beer’s Law & Colorimetry PowerPoint Presentation, free download Beer's Law Temperature In other words, a solution. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Beer's law states that a chemical solution's concentration is directly. Beer's Law Temperature.
From www.studocu.com
Chem 181 4 Beer's Law Fall 2020 Determining the Concentration of a Beer's Law Temperature Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. The premise is that a light beam becomes weaker as.. Beer's Law Temperature.
From sciencenotes.org
Beer's Law Equation and Example Beer's Law Temperature Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance.. Beer's Law Temperature.