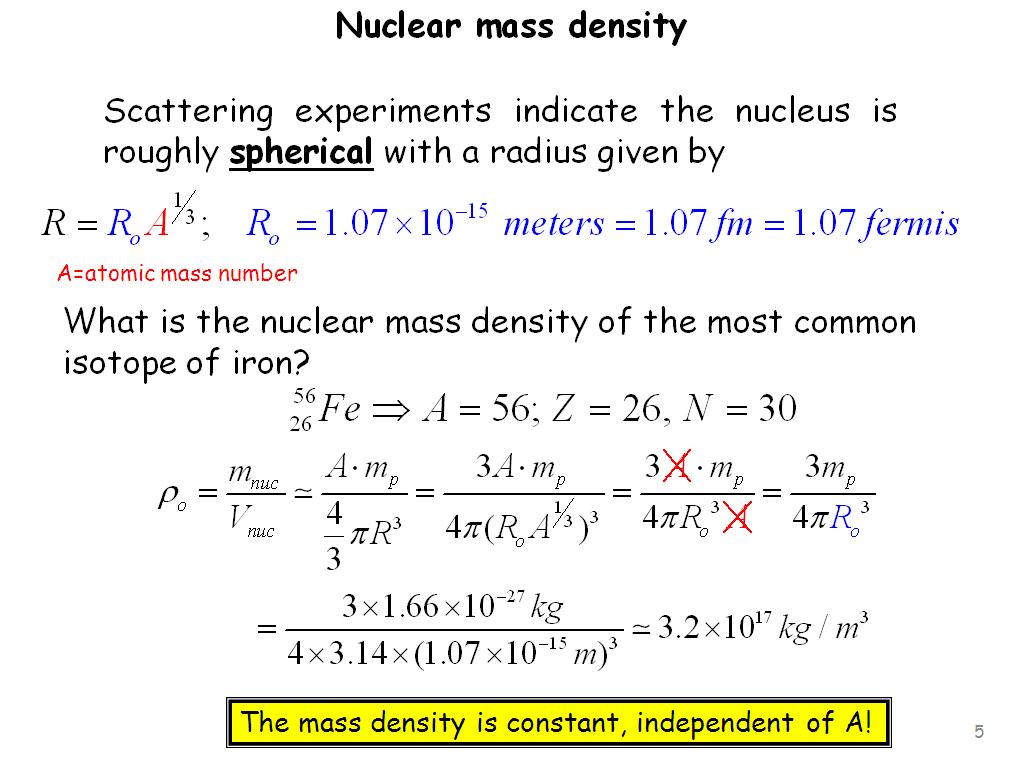
How To Calculate Density Of A Nucleus . For example, natural uranium consists primarily of isotope. Calculate the approximate density of a lithium nucleus. (see figure \ (\pageindex {1}\).). Calculate the density of the nucleus. Why are some nuclei stable while others decay? The nuclear density for a typical nucleus can be approximately calculated from. Label nuclear states with the nuclear spin and parity quantum numbers. 0+ (j = 0, parity even), 2− (j = 2, parity odd) the parity of a nucleus is given by the product of the parities of all. To find the approximate density of this nucleus, assume the nucleus is spherical. Calculate its volume using the radius found in part (a), and then find its density from \(\rho = m/v\). Density = mass / volume. Density is proprtional to a/r3. What is inside the nucleus? Assume the atomic mass of lithium to be 7u. Nuclear density is the density of the nucleus of an atom, averaging about 4 ⋅ 1017kg/m3 4 ⋅ 10 17 k g / m 3.
from ramsayfarrah.blogspot.com
To find the approximate density of this nucleus, assume the nucleus is spherical. The nuclear density for a typical nucleus can be approximately calculated from the size of the nucleus and its mass. Nuclear density is the density of the nucleus of an atom, averaging about 4 ⋅ 1017kg/m3 4 ⋅ 10 17 k g / m 3. Why are some nuclei stable while others decay? What is inside the nucleus? Label nuclear states with the nuclear spin and parity quantum numbers. Calculate the density of the nucleus. Calculate the approximate density of a lithium nucleus. Calculate its volume using the radius found in part (a), and then find its density from \(\rho = m/v\). For example, natural uranium consists primarily of isotope.
Nuclear density formula RamsayFarrah
How To Calculate Density Of A Nucleus Nuclear density is the density of the nucleus of an atom, averaging about 4 ⋅ 1017kg/m3 4 ⋅ 10 17 k g / m 3. Label nuclear states with the nuclear spin and parity quantum numbers. To find the approximate density of this nucleus, assume the nucleus is spherical. Calculate its volume using the radius found in part (a), and then find its density from \(\rho = m/v\). The nuclear density for a typical nucleus can be approximately calculated from the size of the nucleus and its mass. What is inside the nucleus? Density is proprtional to a/r3. Density = mass / volume. Why are some nuclei stable while others decay? 0+ (j = 0, parity even), 2− (j = 2, parity odd) the parity of a nucleus is given by the product of the parities of all. Assume the atomic mass of lithium to be 7u. Calculate the density of the nucleus. The nuclear density for a typical nucleus can be approximately calculated from. Calculate the approximate density of a lithium nucleus. (see figure \ (\pageindex {1}\).). Nuclear density is the density of the nucleus of an atom, averaging about 4 ⋅ 1017kg/m3 4 ⋅ 10 17 k g / m 3.
From byjus.com
Density of an nucleus How To Calculate Density Of A Nucleus The nuclear density for a typical nucleus can be approximately calculated from. Calculate the density of the nucleus. Label nuclear states with the nuclear spin and parity quantum numbers. 0+ (j = 0, parity even), 2− (j = 2, parity odd) the parity of a nucleus is given by the product of the parities of all. Nuclear density is the. How To Calculate Density Of A Nucleus.
From mungfali.com
Periodic Table With Nucleon Number How To Calculate Density Of A Nucleus To find the approximate density of this nucleus, assume the nucleus is spherical. (see figure \ (\pageindex {1}\).). Density = mass / volume. The nuclear density for a typical nucleus can be approximately calculated from the size of the nucleus and its mass. Nuclear density is the density of the nucleus of an atom, averaging about 4 ⋅ 1017kg/m3 4. How To Calculate Density Of A Nucleus.
From www.cyberphysics.co.uk
The nucleus How To Calculate Density Of A Nucleus Calculate the approximate density of a lithium nucleus. Why are some nuclei stable while others decay? Calculate the density of the nucleus. The nuclear density for a typical nucleus can be approximately calculated from the size of the nucleus and its mass. Nuclear density is the density of the nucleus of an atom, averaging about 4 ⋅ 1017kg/m3 4 ⋅. How To Calculate Density Of A Nucleus.
From byjus.com
The density of a nucleus in which mass of each nucleon is 1.6710 to the How To Calculate Density Of A Nucleus Density is proprtional to a/r3. Assume the atomic mass of lithium to be 7u. What is inside the nucleus? Calculate its volume using the radius found in part (a), and then find its density from \(\rho = m/v\). (see figure \ (\pageindex {1}\).). Calculate the density of the nucleus. For example, natural uranium consists primarily of isotope. Calculate the approximate. How To Calculate Density Of A Nucleus.
From www.chegg.com
Solved EXAMPLE Calculate the nuclear matter density at the How To Calculate Density Of A Nucleus The nuclear density for a typical nucleus can be approximately calculated from the size of the nucleus and its mass. Calculate its volume using the radius found in part (a), and then find its density from \(\rho = m/v\). For example, natural uranium consists primarily of isotope. Nuclear density is the density of the nucleus of an atom, averaging about. How To Calculate Density Of A Nucleus.
From www.youtube.com
The density of nucleus is about………….times the density of atom. YouTube How To Calculate Density Of A Nucleus 0+ (j = 0, parity even), 2− (j = 2, parity odd) the parity of a nucleus is given by the product of the parities of all. What is inside the nucleus? The nuclear density for a typical nucleus can be approximately calculated from. For example, natural uranium consists primarily of isotope. Nuclear density is the density of the nucleus. How To Calculate Density Of A Nucleus.
From www.vrogue.co
Solved Calculating Partieles In The Nucleus Name Atom vrogue.co How To Calculate Density Of A Nucleus Calculate the approximate density of a lithium nucleus. What is inside the nucleus? Calculate its volume using the radius found in part (a), and then find its density from \(\rho = m/v\). Nuclear density is the density of the nucleus of an atom, averaging about 4 ⋅ 1017kg/m3 4 ⋅ 10 17 k g / m 3. Calculate the density. How To Calculate Density Of A Nucleus.
From www.chegg.com
Solved EXAMPLE Calculate the nuclear matter density at the How To Calculate Density Of A Nucleus Why are some nuclei stable while others decay? Nuclear density is the density of the nucleus of an atom, averaging about 4 ⋅ 1017kg/m3 4 ⋅ 10 17 k g / m 3. Density = mass / volume. For example, natural uranium consists primarily of isotope. The nuclear density for a typical nucleus can be approximately calculated from. The nuclear. How To Calculate Density Of A Nucleus.
From www.toppr.com
The density of a nucleus in which mass of each nucle on is 1.67 × 10 How To Calculate Density Of A Nucleus What is inside the nucleus? Calculate the density of the nucleus. Calculate its volume using the radius found in part (a), and then find its density from \(\rho = m/v\). 0+ (j = 0, parity even), 2− (j = 2, parity odd) the parity of a nucleus is given by the product of the parities of all. The nuclear density. How To Calculate Density Of A Nucleus.
From www.youtube.com
Nuclear size and density Nuclei Physics Khan Academy YouTube How To Calculate Density Of A Nucleus (see figure \ (\pageindex {1}\).). Calculate the density of the nucleus. 0+ (j = 0, parity even), 2− (j = 2, parity odd) the parity of a nucleus is given by the product of the parities of all. To find the approximate density of this nucleus, assume the nucleus is spherical. For example, natural uranium consists primarily of isotope. Calculate. How To Calculate Density Of A Nucleus.
From www.youtube.com
13.18 How to calculate the energy released in a nuclear reaction YouTube How To Calculate Density Of A Nucleus Calculate the density of the nucleus. Calculate the approximate density of a lithium nucleus. The nuclear density for a typical nucleus can be approximately calculated from. (see figure \ (\pageindex {1}\).). Density is proprtional to a/r3. Assume the atomic mass of lithium to be 7u. For example, natural uranium consists primarily of isotope. To find the approximate density of this. How To Calculate Density Of A Nucleus.
From www.slideserve.com
PPT Nuclear Radius PowerPoint Presentation, free download ID2964639 How To Calculate Density Of A Nucleus Label nuclear states with the nuclear spin and parity quantum numbers. Why are some nuclei stable while others decay? Calculate the approximate density of a lithium nucleus. The nuclear density for a typical nucleus can be approximately calculated from. Calculate its volume using the radius found in part (a), and then find its density from \(\rho = m/v\). To find. How To Calculate Density Of A Nucleus.
From www.teachoo.com
Nucleons, Atomic Number and Mass Number Definitions.. and more How To Calculate Density Of A Nucleus What is inside the nucleus? The nuclear density for a typical nucleus can be approximately calculated from. For example, natural uranium consists primarily of isotope. To find the approximate density of this nucleus, assume the nucleus is spherical. Calculate its volume using the radius found in part (a), and then find its density from \(\rho = m/v\). Density = mass. How To Calculate Density Of A Nucleus.
From mrmanojpandey.com
Shape, Size Of Nucleus And Nuclear Density » Maths And Physics With How To Calculate Density Of A Nucleus Density = mass / volume. Label nuclear states with the nuclear spin and parity quantum numbers. Assume the atomic mass of lithium to be 7u. The nuclear density for a typical nucleus can be approximately calculated from. Calculate the density of the nucleus. Nuclear density is the density of the nucleus of an atom, averaging about 4 ⋅ 1017kg/m3 4. How To Calculate Density Of A Nucleus.
From www.youtube.com
NUCLEAR DENSITY / HOW TO CALCULATE THE DENSITY OF A NUCLEUS YouTube How To Calculate Density Of A Nucleus Label nuclear states with the nuclear spin and parity quantum numbers. What is inside the nucleus? Calculate the approximate density of a lithium nucleus. Density = mass / volume. (see figure \ (\pageindex {1}\).). 0+ (j = 0, parity even), 2− (j = 2, parity odd) the parity of a nucleus is given by the product of the parities of. How To Calculate Density Of A Nucleus.
From general.chemistrysteps.com
Nuclear Binding Energy Chemistry Steps How To Calculate Density Of A Nucleus To find the approximate density of this nucleus, assume the nucleus is spherical. The nuclear density for a typical nucleus can be approximately calculated from. Calculate the approximate density of a lithium nucleus. Nuclear density is the density of the nucleus of an atom, averaging about 4 ⋅ 1017kg/m3 4 ⋅ 10 17 k g / m 3. Why are. How To Calculate Density Of A Nucleus.
From www.toppr.com
The radius of a nucleus of nucleon number 16 is 3 × 10^15m. What is the How To Calculate Density Of A Nucleus Density = mass / volume. Nuclear density is the density of the nucleus of an atom, averaging about 4 ⋅ 1017kg/m3 4 ⋅ 10 17 k g / m 3. What is inside the nucleus? (see figure \ (\pageindex {1}\).). For example, natural uranium consists primarily of isotope. Label nuclear states with the nuclear spin and parity quantum numbers. Calculate. How To Calculate Density Of A Nucleus.
From www.slideserve.com
PPT Section 4.2—Atomic Structure PowerPoint Presentation, free How To Calculate Density Of A Nucleus Why are some nuclei stable while others decay? Calculate its volume using the radius found in part (a), and then find its density from \(\rho = m/v\). Nuclear density is the density of the nucleus of an atom, averaging about 4 ⋅ 1017kg/m3 4 ⋅ 10 17 k g / m 3. Density = mass / volume. The nuclear density. How To Calculate Density Of A Nucleus.
From www.toppr.com
Assuming the radius of a nucleus to be equal to R = 0.13 sqrt[3]{A} ,pm How To Calculate Density Of A Nucleus Calculate the approximate density of a lithium nucleus. To find the approximate density of this nucleus, assume the nucleus is spherical. Why are some nuclei stable while others decay? Assume the atomic mass of lithium to be 7u. (see figure \ (\pageindex {1}\).). For example, natural uranium consists primarily of isotope. Label nuclear states with the nuclear spin and parity. How To Calculate Density Of A Nucleus.
From www.toppr.com
The nucleus of an atom can be modeled as several protons and neutrons How To Calculate Density Of A Nucleus 0+ (j = 0, parity even), 2− (j = 2, parity odd) the parity of a nucleus is given by the product of the parities of all. To find the approximate density of this nucleus, assume the nucleus is spherical. Assume the atomic mass of lithium to be 7u. Calculate the approximate density of a lithium nucleus. For example, natural. How To Calculate Density Of A Nucleus.
From sciencenotes.org
Mass Defect Definition and Formula How To Calculate Density Of A Nucleus What is inside the nucleus? Calculate the density of the nucleus. Density = mass / volume. 0+ (j = 0, parity even), 2− (j = 2, parity odd) the parity of a nucleus is given by the product of the parities of all. Why are some nuclei stable while others decay? (see figure \ (\pageindex {1}\).). For example, natural uranium. How To Calculate Density Of A Nucleus.
From byjus.com
Which of the following has maximum density of nucleus. (1)Si (z=14,A=30 How To Calculate Density Of A Nucleus Calculate the density of the nucleus. Density is proprtional to a/r3. Why are some nuclei stable while others decay? Assume the atomic mass of lithium to be 7u. What is inside the nucleus? (see figure \ (\pageindex {1}\).). The nuclear density for a typical nucleus can be approximately calculated from the size of the nucleus and its mass. Nuclear density. How To Calculate Density Of A Nucleus.
From www.youtube.com
A Level Physics density of nuclei YouTube How To Calculate Density Of A Nucleus Nuclear density is the density of the nucleus of an atom, averaging about 4 ⋅ 1017kg/m3 4 ⋅ 10 17 k g / m 3. To find the approximate density of this nucleus, assume the nucleus is spherical. The nuclear density for a typical nucleus can be approximately calculated from. For example, natural uranium consists primarily of isotope. What is. How To Calculate Density Of A Nucleus.
From brainly.in
1. Find the density of F Nucleus 2. Find the density of Nucleus having How To Calculate Density Of A Nucleus Label nuclear states with the nuclear spin and parity quantum numbers. To find the approximate density of this nucleus, assume the nucleus is spherical. Density = mass / volume. Calculate its volume using the radius found in part (a), and then find its density from \(\rho = m/v\). Density is proprtional to a/r3. Assume the atomic mass of lithium to. How To Calculate Density Of A Nucleus.
From mrmanojpandey.com
Shape, Size Of Nucleus And Nuclear Density » Maths And Physics With How To Calculate Density Of A Nucleus Nuclear density is the density of the nucleus of an atom, averaging about 4 ⋅ 1017kg/m3 4 ⋅ 10 17 k g / m 3. 0+ (j = 0, parity even), 2− (j = 2, parity odd) the parity of a nucleus is given by the product of the parities of all. What is inside the nucleus? For example, natural. How To Calculate Density Of A Nucleus.
From www.nuclear-power.com
Atomic and Nuclear Structure Definition & Characteristics nuclear How To Calculate Density Of A Nucleus For example, natural uranium consists primarily of isotope. 0+ (j = 0, parity even), 2− (j = 2, parity odd) the parity of a nucleus is given by the product of the parities of all. Nuclear density is the density of the nucleus of an atom, averaging about 4 ⋅ 1017kg/m3 4 ⋅ 10 17 k g / m 3.. How To Calculate Density Of A Nucleus.
From www.youtube.com
Find the density of `._(6)^(12)C` nucleus. Take atomic mass of `._(6 How To Calculate Density Of A Nucleus The nuclear density for a typical nucleus can be approximately calculated from. Density is proprtional to a/r3. Density = mass / volume. To find the approximate density of this nucleus, assume the nucleus is spherical. Calculate its volume using the radius found in part (a), and then find its density from \(\rho = m/v\). The nuclear density for a typical. How To Calculate Density Of A Nucleus.
From www.youtube.com
The mass density of a nucleus varies with mass number `A` as YouTube How To Calculate Density Of A Nucleus (see figure \ (\pageindex {1}\).). Calculate its volume using the radius found in part (a), and then find its density from \(\rho = m/v\). Why are some nuclei stable while others decay? For example, natural uranium consists primarily of isotope. Nuclear density is the density of the nucleus of an atom, averaging about 4 ⋅ 1017kg/m3 4 ⋅ 10 17. How To Calculate Density Of A Nucleus.
From www.youtube.com
Nuclear Binding Energy Per Nucleon & Mass Defect Problems Nuclear How To Calculate Density Of A Nucleus For example, natural uranium consists primarily of isotope. Label nuclear states with the nuclear spin and parity quantum numbers. Why are some nuclei stable while others decay? The nuclear density for a typical nucleus can be approximately calculated from the size of the nucleus and its mass. (see figure \ (\pageindex {1}\).). Density is proprtional to a/r3. Density = mass. How To Calculate Density Of A Nucleus.
From raimiewojtek.blogspot.com
Nuclear density formula RaimieWojtek How To Calculate Density Of A Nucleus Assume the atomic mass of lithium to be 7u. To find the approximate density of this nucleus, assume the nucleus is spherical. (see figure \ (\pageindex {1}\).). Nuclear density is the density of the nucleus of an atom, averaging about 4 ⋅ 1017kg/m3 4 ⋅ 10 17 k g / m 3. The nuclear density for a typical nucleus can. How To Calculate Density Of A Nucleus.
From byjus.com
which of the following elements has maximum density of nucleus>' n nt1 How To Calculate Density Of A Nucleus The nuclear density for a typical nucleus can be approximately calculated from. Density = mass / volume. Calculate the approximate density of a lithium nucleus. Assume the atomic mass of lithium to be 7u. To find the approximate density of this nucleus, assume the nucleus is spherical. What is inside the nucleus? The nuclear density for a typical nucleus can. How To Calculate Density Of A Nucleus.
From ramsayfarrah.blogspot.com
Nuclear density formula RamsayFarrah How To Calculate Density Of A Nucleus Density = mass / volume. 0+ (j = 0, parity even), 2− (j = 2, parity odd) the parity of a nucleus is given by the product of the parities of all. The nuclear density for a typical nucleus can be approximately calculated from. Why are some nuclei stable while others decay? (see figure \ (\pageindex {1}\).). Assume the atomic. How To Calculate Density Of A Nucleus.
From byjus.com
13. Find nuclear mass density of 238U92. Given R= 1.5fermi and mass of How To Calculate Density Of A Nucleus To find the approximate density of this nucleus, assume the nucleus is spherical. The nuclear density for a typical nucleus can be approximately calculated from the size of the nucleus and its mass. Density = mass / volume. What is inside the nucleus? For example, natural uranium consists primarily of isotope. Nuclear density is the density of the nucleus of. How To Calculate Density Of A Nucleus.
From bejaysamael.blogspot.com
Atomic density calculator BejaySamael How To Calculate Density Of A Nucleus The nuclear density for a typical nucleus can be approximately calculated from the size of the nucleus and its mass. Density = mass / volume. Assume the atomic mass of lithium to be 7u. 0+ (j = 0, parity even), 2− (j = 2, parity odd) the parity of a nucleus is given by the product of the parities of. How To Calculate Density Of A Nucleus.
From www.topperlearning.com
Calculate the binding energy and binding energy per nucleon of 26Fe How To Calculate Density Of A Nucleus Calculate the density of the nucleus. Why are some nuclei stable while others decay? Nuclear density is the density of the nucleus of an atom, averaging about 4 ⋅ 1017kg/m3 4 ⋅ 10 17 k g / m 3. Assume the atomic mass of lithium to be 7u. The nuclear density for a typical nucleus can be approximately calculated from. How To Calculate Density Of A Nucleus.