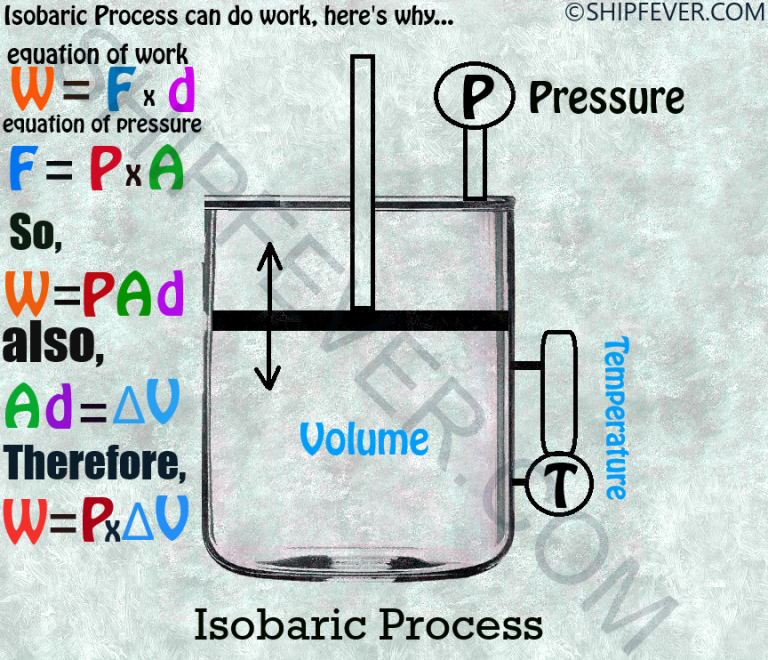
Isobaric Process Of Water Boiling . An example of the isobaric process includes the boiling of water to steam or the freezing of water to ice. What are are boiling of water and freezing of water categorized in with relevance to the 1st law of thermodynamics? During an isobaric process, any heat added to the system will result in an increase in volume if the system does work on its surroundings. The pressure in the cylinder. In the process, a gas either expands or contracts to maintain constant pressure,. The boiling of water in a pressure cooker is not an example of the isobaric process as pressure can’t remain. A quintessential example of an isobaric process is boiling water in an open vessel at a certain elevation, where the water goes from a liquid to. One common example of an isobaric process is the expansion of gas in an engine piston. Both boiling of water and freezing of water are examples of isobaric processes.
from shipfever.com
The boiling of water in a pressure cooker is not an example of the isobaric process as pressure can’t remain. An example of the isobaric process includes the boiling of water to steam or the freezing of water to ice. One common example of an isobaric process is the expansion of gas in an engine piston. Both boiling of water and freezing of water are examples of isobaric processes. In the process, a gas either expands or contracts to maintain constant pressure,. The pressure in the cylinder. A quintessential example of an isobaric process is boiling water in an open vessel at a certain elevation, where the water goes from a liquid to. What are are boiling of water and freezing of water categorized in with relevance to the 1st law of thermodynamics? During an isobaric process, any heat added to the system will result in an increase in volume if the system does work on its surroundings.
Learn First Law of Thermodynamics in Simple Language ShipFever
Isobaric Process Of Water Boiling What are are boiling of water and freezing of water categorized in with relevance to the 1st law of thermodynamics? The pressure in the cylinder. Both boiling of water and freezing of water are examples of isobaric processes. In the process, a gas either expands or contracts to maintain constant pressure,. The boiling of water in a pressure cooker is not an example of the isobaric process as pressure can’t remain. An example of the isobaric process includes the boiling of water to steam or the freezing of water to ice. A quintessential example of an isobaric process is boiling water in an open vessel at a certain elevation, where the water goes from a liquid to. One common example of an isobaric process is the expansion of gas in an engine piston. What are are boiling of water and freezing of water categorized in with relevance to the 1st law of thermodynamics? During an isobaric process, any heat added to the system will result in an increase in volume if the system does work on its surroundings.
From www.researchgate.net
Heat Engine with Isobaric Process. Top left figure shows the initial Isobaric Process Of Water Boiling During an isobaric process, any heat added to the system will result in an increase in volume if the system does work on its surroundings. The boiling of water in a pressure cooker is not an example of the isobaric process as pressure can’t remain. The pressure in the cylinder. What are are boiling of water and freezing of water. Isobaric Process Of Water Boiling.
From www.researchgate.net
Schematics of the isobaric setup. Download Scientific Diagram Isobaric Process Of Water Boiling The boiling of water in a pressure cooker is not an example of the isobaric process as pressure can’t remain. A quintessential example of an isobaric process is boiling water in an open vessel at a certain elevation, where the water goes from a liquid to. During an isobaric process, any heat added to the system will result in an. Isobaric Process Of Water Boiling.
From www.tec-science.com
Isobaric process in a closed system tecscience Isobaric Process Of Water Boiling During an isobaric process, any heat added to the system will result in an increase in volume if the system does work on its surroundings. One common example of an isobaric process is the expansion of gas in an engine piston. In the process, a gas either expands or contracts to maintain constant pressure,. The boiling of water in a. Isobaric Process Of Water Boiling.
From www.slideserve.com
PPT Thermodynamics PowerPoint Presentation, free download ID3150253 Isobaric Process Of Water Boiling Both boiling of water and freezing of water are examples of isobaric processes. In the process, a gas either expands or contracts to maintain constant pressure,. A quintessential example of an isobaric process is boiling water in an open vessel at a certain elevation, where the water goes from a liquid to. An example of the isobaric process includes the. Isobaric Process Of Water Boiling.
From ar.inspiredpencil.com
Isobaric Process Isobaric Process Of Water Boiling Both boiling of water and freezing of water are examples of isobaric processes. The boiling of water in a pressure cooker is not an example of the isobaric process as pressure can’t remain. In the process, a gas either expands or contracts to maintain constant pressure,. The pressure in the cylinder. A quintessential example of an isobaric process is boiling. Isobaric Process Of Water Boiling.
From slideplayer.com
Laws of Thermodynamics ppt download Isobaric Process Of Water Boiling A quintessential example of an isobaric process is boiling water in an open vessel at a certain elevation, where the water goes from a liquid to. The boiling of water in a pressure cooker is not an example of the isobaric process as pressure can’t remain. During an isobaric process, any heat added to the system will result in an. Isobaric Process Of Water Boiling.
From app.jove.com
Isochoric and Isobaric Processes Concept Physics JoVe Isobaric Process Of Water Boiling What are are boiling of water and freezing of water categorized in with relevance to the 1st law of thermodynamics? During an isobaric process, any heat added to the system will result in an increase in volume if the system does work on its surroundings. Both boiling of water and freezing of water are examples of isobaric processes. A quintessential. Isobaric Process Of Water Boiling.
From design1systems.com
Understanding Isobaric Processes with PV Diagrams A Comprehensive Guide Isobaric Process Of Water Boiling An example of the isobaric process includes the boiling of water to steam or the freezing of water to ice. Both boiling of water and freezing of water are examples of isobaric processes. A quintessential example of an isobaric process is boiling water in an open vessel at a certain elevation, where the water goes from a liquid to. During. Isobaric Process Of Water Boiling.
From www.slideshare.net
Chapter 15=Thermodynamics Isobaric Process Of Water Boiling One common example of an isobaric process is the expansion of gas in an engine piston. The boiling of water in a pressure cooker is not an example of the isobaric process as pressure can’t remain. An example of the isobaric process includes the boiling of water to steam or the freezing of water to ice. What are are boiling. Isobaric Process Of Water Boiling.
From www.youtube.com
Isobaric Process (Basics, pV diagram, Work Done, Change in Internal Isobaric Process Of Water Boiling One common example of an isobaric process is the expansion of gas in an engine piston. In the process, a gas either expands or contracts to maintain constant pressure,. During an isobaric process, any heat added to the system will result in an increase in volume if the system does work on its surroundings. Both boiling of water and freezing. Isobaric Process Of Water Boiling.
From www.etutorworld.com
Isobaric Process eTutorWorld Isobaric Process Of Water Boiling In the process, a gas either expands or contracts to maintain constant pressure,. What are are boiling of water and freezing of water categorized in with relevance to the 1st law of thermodynamics? Both boiling of water and freezing of water are examples of isobaric processes. The pressure in the cylinder. The boiling of water in a pressure cooker is. Isobaric Process Of Water Boiling.
From www.slideserve.com
PPT THERMODYNAMIC PowerPoint Presentation, free download ID6381537 Isobaric Process Of Water Boiling In the process, a gas either expands or contracts to maintain constant pressure,. The pressure in the cylinder. An example of the isobaric process includes the boiling of water to steam or the freezing of water to ice. A quintessential example of an isobaric process is boiling water in an open vessel at a certain elevation, where the water goes. Isobaric Process Of Water Boiling.
From www.slideserve.com
PPT Do Now PowerPoint Presentation, free download ID6329644 Isobaric Process Of Water Boiling What are are boiling of water and freezing of water categorized in with relevance to the 1st law of thermodynamics? In the process, a gas either expands or contracts to maintain constant pressure,. An example of the isobaric process includes the boiling of water to steam or the freezing of water to ice. Both boiling of water and freezing of. Isobaric Process Of Water Boiling.
From www.slideserve.com
PPT THERMODYNAMIC PowerPoint Presentation, free download ID9604221 Isobaric Process Of Water Boiling An example of the isobaric process includes the boiling of water to steam or the freezing of water to ice. What are are boiling of water and freezing of water categorized in with relevance to the 1st law of thermodynamics? A quintessential example of an isobaric process is boiling water in an open vessel at a certain elevation, where the. Isobaric Process Of Water Boiling.
From www.tec-science.com
Isobaric process in a closed system tecscience Isobaric Process Of Water Boiling The pressure in the cylinder. One common example of an isobaric process is the expansion of gas in an engine piston. During an isobaric process, any heat added to the system will result in an increase in volume if the system does work on its surroundings. In the process, a gas either expands or contracts to maintain constant pressure,. A. Isobaric Process Of Water Boiling.
From www.tec-science.com
Isobaric process in a closed system tecscience Isobaric Process Of Water Boiling The boiling of water in a pressure cooker is not an example of the isobaric process as pressure can’t remain. One common example of an isobaric process is the expansion of gas in an engine piston. In the process, a gas either expands or contracts to maintain constant pressure,. Both boiling of water and freezing of water are examples of. Isobaric Process Of Water Boiling.
From www.youtube.com
ISOTHERMAL PROCESS ADIABATIC PROCESS ISOCHORIC PROCESS ISOBARIC Isobaric Process Of Water Boiling In the process, a gas either expands or contracts to maintain constant pressure,. What are are boiling of water and freezing of water categorized in with relevance to the 1st law of thermodynamics? The pressure in the cylinder. During an isobaric process, any heat added to the system will result in an increase in volume if the system does work. Isobaric Process Of Water Boiling.
From www.chegg.com
Solved FIGURE P6.57 6.58 Water at 100 kPa, 25°C is brought Isobaric Process Of Water Boiling Both boiling of water and freezing of water are examples of isobaric processes. The pressure in the cylinder. An example of the isobaric process includes the boiling of water to steam or the freezing of water to ice. During an isobaric process, any heat added to the system will result in an increase in volume if the system does work. Isobaric Process Of Water Boiling.
From www.etutorworld.com
Isobaric Process eTutorWorld Isobaric Process Of Water Boiling The boiling of water in a pressure cooker is not an example of the isobaric process as pressure can’t remain. What are are boiling of water and freezing of water categorized in with relevance to the 1st law of thermodynamics? During an isobaric process, any heat added to the system will result in an increase in volume if the system. Isobaric Process Of Water Boiling.
From www.youtube.com
Isobaric Process Thermodynamics Work Done by the Gas PV Diagram Isobaric Process Of Water Boiling A quintessential example of an isobaric process is boiling water in an open vessel at a certain elevation, where the water goes from a liquid to. The pressure in the cylinder. Both boiling of water and freezing of water are examples of isobaric processes. One common example of an isobaric process is the expansion of gas in an engine piston.. Isobaric Process Of Water Boiling.
From www.youtube.com
liquid solution 08 Isobaric fractional distillation, boiling point Isobaric Process Of Water Boiling What are are boiling of water and freezing of water categorized in with relevance to the 1st law of thermodynamics? Both boiling of water and freezing of water are examples of isobaric processes. During an isobaric process, any heat added to the system will result in an increase in volume if the system does work on its surroundings. An example. Isobaric Process Of Water Boiling.
From www.slideserve.com
PPT THERMODYNAMIC PowerPoint Presentation, free download ID9604221 Isobaric Process Of Water Boiling Both boiling of water and freezing of water are examples of isobaric processes. One common example of an isobaric process is the expansion of gas in an engine piston. The pressure in the cylinder. The boiling of water in a pressure cooker is not an example of the isobaric process as pressure can’t remain. A quintessential example of an isobaric. Isobaric Process Of Water Boiling.
From mech-engineeringbd.blogspot.com
Isobaric Process (Constant Pressure Process) Mechanical Engineering Isobaric Process Of Water Boiling During an isobaric process, any heat added to the system will result in an increase in volume if the system does work on its surroundings. The pressure in the cylinder. One common example of an isobaric process is the expansion of gas in an engine piston. The boiling of water in a pressure cooker is not an example of the. Isobaric Process Of Water Boiling.
From www.youtube.com
Isobaric Process Thermodynamics Work & Heat Energy, Molar Heat Isobaric Process Of Water Boiling Both boiling of water and freezing of water are examples of isobaric processes. During an isobaric process, any heat added to the system will result in an increase in volume if the system does work on its surroundings. An example of the isobaric process includes the boiling of water to steam or the freezing of water to ice. The pressure. Isobaric Process Of Water Boiling.
From shipfever.com
Learn First Law of Thermodynamics in Simple Language ShipFever Isobaric Process Of Water Boiling What are are boiling of water and freezing of water categorized in with relevance to the 1st law of thermodynamics? The boiling of water in a pressure cooker is not an example of the isobaric process as pressure can’t remain. One common example of an isobaric process is the expansion of gas in an engine piston. During an isobaric process,. Isobaric Process Of Water Boiling.
From design1systems.com
Understanding Isobaric Processes with PV Diagrams A Comprehensive Guide Isobaric Process Of Water Boiling What are are boiling of water and freezing of water categorized in with relevance to the 1st law of thermodynamics? During an isobaric process, any heat added to the system will result in an increase in volume if the system does work on its surroundings. The pressure in the cylinder. An example of the isobaric process includes the boiling of. Isobaric Process Of Water Boiling.
From www.tec-science.com
Isobaric process in a closed system tecscience Isobaric Process Of Water Boiling The pressure in the cylinder. In the process, a gas either expands or contracts to maintain constant pressure,. Both boiling of water and freezing of water are examples of isobaric processes. What are are boiling of water and freezing of water categorized in with relevance to the 1st law of thermodynamics? The boiling of water in a pressure cooker is. Isobaric Process Of Water Boiling.
From www.scribd.com
Isobaric Process PDF Enthalpy Steam Isobaric Process Of Water Boiling The boiling of water in a pressure cooker is not an example of the isobaric process as pressure can’t remain. During an isobaric process, any heat added to the system will result in an increase in volume if the system does work on its surroundings. One common example of an isobaric process is the expansion of gas in an engine. Isobaric Process Of Water Boiling.
From www.numerade.com
SOLVEDWater at 100 kPa, 25^∘ C is brought to the boiling point in a Isobaric Process Of Water Boiling Both boiling of water and freezing of water are examples of isobaric processes. The boiling of water in a pressure cooker is not an example of the isobaric process as pressure can’t remain. What are are boiling of water and freezing of water categorized in with relevance to the 1st law of thermodynamics? An example of the isobaric process includes. Isobaric Process Of Water Boiling.
From www.slideserve.com
PPT Work in Thermodynamic Processes PowerPoint Presentation, free Isobaric Process Of Water Boiling An example of the isobaric process includes the boiling of water to steam or the freezing of water to ice. Both boiling of water and freezing of water are examples of isobaric processes. A quintessential example of an isobaric process is boiling water in an open vessel at a certain elevation, where the water goes from a liquid to. The. Isobaric Process Of Water Boiling.
From lawofthermodynamicsinfo.com
Thermodynamic Process (With Examples) Isobaric, Isochoric, Adiabatic Isobaric Process Of Water Boiling In the process, a gas either expands or contracts to maintain constant pressure,. One common example of an isobaric process is the expansion of gas in an engine piston. During an isobaric process, any heat added to the system will result in an increase in volume if the system does work on its surroundings. An example of the isobaric process. Isobaric Process Of Water Boiling.
From slideplayer.com
Laws of Thermodynamics ppt download Isobaric Process Of Water Boiling During an isobaric process, any heat added to the system will result in an increase in volume if the system does work on its surroundings. A quintessential example of an isobaric process is boiling water in an open vessel at a certain elevation, where the water goes from a liquid to. One common example of an isobaric process is the. Isobaric Process Of Water Boiling.
From www.eigenplus.com
Fundamentals Of Isobaric Process Key Concepts, Work Done Isobaric Process Of Water Boiling The pressure in the cylinder. In the process, a gas either expands or contracts to maintain constant pressure,. A quintessential example of an isobaric process is boiling water in an open vessel at a certain elevation, where the water goes from a liquid to. One common example of an isobaric process is the expansion of gas in an engine piston.. Isobaric Process Of Water Boiling.
From www.chemistrylearner.com
Physical Chemistry Chemistry Learner Isobaric Process Of Water Boiling The boiling of water in a pressure cooker is not an example of the isobaric process as pressure can’t remain. In the process, a gas either expands or contracts to maintain constant pressure,. An example of the isobaric process includes the boiling of water to steam or the freezing of water to ice. The pressure in the cylinder. During an. Isobaric Process Of Water Boiling.
From www.youtube.com
Thermodynamic Processes Isobaric, Isochoric, Isothermal and Adiabatic Isobaric Process Of Water Boiling What are are boiling of water and freezing of water categorized in with relevance to the 1st law of thermodynamics? Both boiling of water and freezing of water are examples of isobaric processes. A quintessential example of an isobaric process is boiling water in an open vessel at a certain elevation, where the water goes from a liquid to. During. Isobaric Process Of Water Boiling.