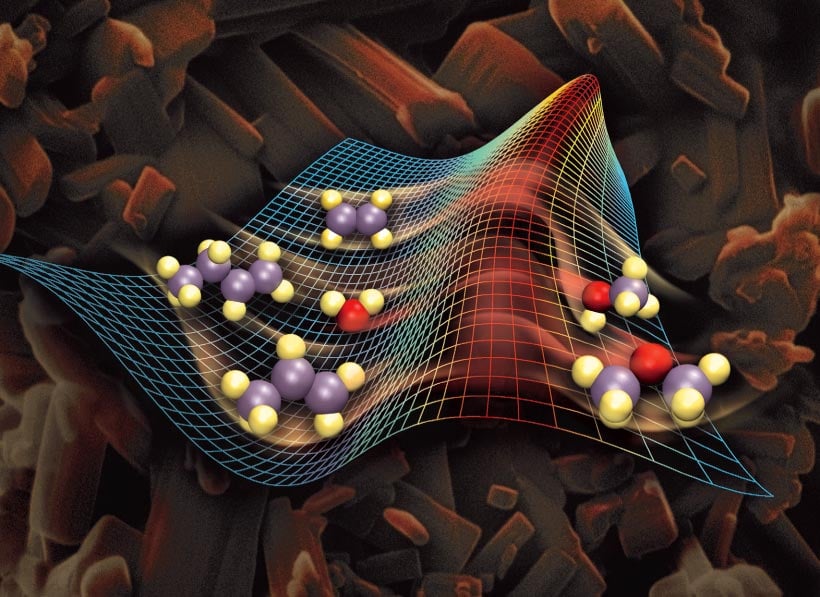
Catalyst In Chemistry Example . catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Adding a drop of blood to. gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within the gas or. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. In heterogeneous catalysis, catalysts provide a surface. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; types of catalytic reactions. usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its. In a heterogeneous reaction, the.
from scitechdaily.com
gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within the gas or. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. types of catalytic reactions. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; In heterogeneous catalysis, catalysts provide a surface. In a heterogeneous reaction, the. Adding a drop of blood to.
Science Made Simple What Are Catalysts?
Catalyst In Chemistry Example usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its. In heterogeneous catalysis, catalysts provide a surface. gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within the gas or. types of catalytic reactions. In a heterogeneous reaction, the. usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Adding a drop of blood to. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids;
From fity.club
Enzyme The Catalyst Catalyst In Chemistry Example catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within the gas or. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids;. Catalyst In Chemistry Example.
From medicaltaste.weebly.com
Periodic table catalyst definition chemistry medicaltaste Catalyst In Chemistry Example types of catalytic reactions. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its. catalysts allow a reaction to proceed via a. Catalyst In Chemistry Example.
From news.emory.edu
Chemical Catalyst Turns 'Trash' to 'Treasure' Catalyst In Chemistry Example In a heterogeneous reaction, the. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within the gas or. In heterogeneous catalysis, catalysts provide a surface. types of catalytic reactions. Adding a drop. Catalyst In Chemistry Example.
From exojqwcbb.blob.core.windows.net
Catalyst Chemistry Symbol at Brenda Jones blog Catalyst In Chemistry Example catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within the gas or. usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst. Catalyst In Chemistry Example.
From brainly.in
define catalyst with example ?? Brainly.in Catalyst In Chemistry Example catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. In heterogeneous catalysis, catalysts provide a surface. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. In a heterogeneous reaction, the. usually when someone refers to a catalyst, they mean a. Catalyst In Chemistry Example.
From schoolbag.info
A catalyst speeds up a reaction by providing the reactants with an Catalyst In Chemistry Example Adding a drop of blood to. gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within the gas or. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. the most effective catalyst of all is the enzyme catalase,. Catalyst In Chemistry Example.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalyst In Chemistry Example catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its. gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface. Catalyst In Chemistry Example.
From exohmhkon.blob.core.windows.net
Catalyst Year Meaning at Wayne Wang blog Catalyst In Chemistry Example gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within the gas or. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up. Catalyst In Chemistry Example.
From www.dreamstime.com
Catalyst Surface with Catalytic Reaction Stock Vector Illustration of Catalyst In Chemistry Example gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within the gas or. In a heterogeneous reaction, the. usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its. catalysts allow. Catalyst In Chemistry Example.
From www.mdpi.com
Catalysts Free FullText Green Chemistry in Organic Synthesis Catalyst In Chemistry Example types of catalytic reactions. gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within the gas or. In heterogeneous catalysis, catalysts provide a surface. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; catalysts allow a reaction to proceed via. Catalyst In Chemistry Example.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalyst In Chemistry Example Adding a drop of blood to. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. In a heterogeneous reaction, the. types of catalytic reactions. usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by. Catalyst In Chemistry Example.
From www.thoughtco.com
Catalysis Definition in Chemistry Catalyst In Chemistry Example In a heterogeneous reaction, the. In heterogeneous catalysis, catalysts provide a surface. gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within the gas or. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. the most effective catalyst of all is. Catalyst In Chemistry Example.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalyst In Chemistry Example In heterogeneous catalysis, catalysts provide a surface. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within the gas or. catalyst, in chemistry, any substance that increases the rate. Catalyst In Chemistry Example.
From phys.org
Researchers help show new way to study and improve catalytic reactions Catalyst In Chemistry Example Adding a drop of blood to. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst. Catalyst In Chemistry Example.
From www.mdpi.com
Catalysts Free FullText Switchable StimuliResponsive Catalyst In Chemistry Example In heterogeneous catalysis, catalysts provide a surface. gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within the gas or. types of catalytic reactions. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. the most effective catalyst of all is. Catalyst In Chemistry Example.
From sheetalschemblog.blogspot.com
Sheetal's Chemistry Blog 6.2.5,6.2.6 and 6.2.7 Catalyst In Chemistry Example the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its. In heterogeneous catalysis, catalysts provide a surface. catalysts allow a reaction to proceed. Catalyst In Chemistry Example.
From blog.syrris.com
Solid phase catalysis in continuous flow Syrris chemistry blog Catalyst In Chemistry Example the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. In a heterogeneous reaction, the. gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather. Catalyst In Chemistry Example.
From exonprxge.blob.core.windows.net
Catalyst Examples In Life at Anthony Snyder blog Catalyst In Chemistry Example catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. In a heterogeneous reaction, the. Adding a drop of blood to. In heterogeneous catalysis, catalysts provide a surface. usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate. Catalyst In Chemistry Example.
From isonlab.wordpress.ncsu.edu
Catalysis/Green Chemistry Ison Research Group Catalyst In Chemistry Example usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its. In heterogeneous catalysis, catalysts provide a surface. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Adding a drop of. Catalyst In Chemistry Example.
From www.youtube.com
Heterogeneous catalyst & catalysis 12th Std Chemistry Science Catalyst In Chemistry Example the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; In heterogeneous catalysis, catalysts provide a surface. Adding a drop of blood to. usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its. In. Catalyst In Chemistry Example.
From www.sciencelearn.org.nz
Chemical reactions and catalysts — Science Learning Hub Catalyst In Chemistry Example Adding a drop of blood to. types of catalytic reactions. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its. catalysts allow. Catalyst In Chemistry Example.
From ar.inspiredpencil.com
Catalyst Examples For Kids Catalyst In Chemistry Example In a heterogeneous reaction, the. Adding a drop of blood to. gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within the gas or. usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction. Catalyst In Chemistry Example.
From www.youtube.com
Catalysts AP Chemistry Khan Academy YouTube Catalyst In Chemistry Example catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within the gas or. In heterogeneous catalysis,. Catalyst In Chemistry Example.
From ar.inspiredpencil.com
Catalyst Chemistry Catalyst In Chemistry Example gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within the gas or. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up. Catalyst In Chemistry Example.
From guidelibdiapedetic.z22.web.core.windows.net
Catalyst Diagram Chemistry Catalyst In Chemistry Example usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its. In a heterogeneous reaction, the. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. In heterogeneous catalysis, catalysts provide a surface. the. Catalyst In Chemistry Example.
From www.youtube.com
What are catalysts? Chemistry for All The Fuse School YouTube Catalyst In Chemistry Example the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; In heterogeneous catalysis, catalysts provide a surface. Adding a drop of blood to. types of catalytic reactions. In a heterogeneous reaction, the. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction.. Catalyst In Chemistry Example.
From study.com
Catalyst Definition, Types & Function Lesson Catalyst In Chemistry Example usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its. In heterogeneous catalysis, catalysts provide a surface. gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within the gas or. . Catalyst In Chemistry Example.
From 2012books.lardbucket.org
Catalysis Catalyst In Chemistry Example usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. catalyst, in chemistry, any substance that increases the rate of. Catalyst In Chemistry Example.
From www.britannica.com
Catalyst Examples, Definition, & Facts Britannica Catalyst In Chemistry Example In a heterogeneous reaction, the. types of catalytic reactions. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; catalysts allow a reaction to proceed via a pathway that has a lower activation energy. Catalyst In Chemistry Example.
From www.mdpi.com
Catalysts Free FullText Functionalization of Ruthenium Olefin Catalyst In Chemistry Example In a heterogeneous reaction, the. Adding a drop of blood to. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within the gas or. catalyst, in chemistry, any substance. Catalyst In Chemistry Example.
From www.mdpi.com
Catalysts Free FullText AirStable Efficient Nickel Catalyst for Catalyst In Chemistry Example catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within the gas or. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids;. Catalyst In Chemistry Example.
From chem.unc.edu
Molecular Catalysts Department of Chemistry Catalyst In Chemistry Example catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within the gas or. In heterogeneous catalysis, catalysts provide a surface. Adding a drop of blood to. catalyst, in chemistry,. Catalyst In Chemistry Example.
From wiredatapoliciaj0.z22.web.core.windows.net
Potential Energy Diagram With Catalyst Catalyst In Chemistry Example catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. types of catalytic reactions. In heterogeneous catalysis, catalysts provide a surface. usually when someone refers to a catalyst, they mean a. Catalyst In Chemistry Example.
From news.virginia.edu
University of Virginia Researchers Uncover New Catalysis Site UVA Today Catalyst In Chemistry Example types of catalytic reactions. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; Adding a drop of blood to. In a heterogeneous reaction, the. gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within the gas or. catalyst, in chemistry,. Catalyst In Chemistry Example.
From scitechdaily.com
Science Made Simple What Are Catalysts? Catalyst In Chemistry Example In a heterogeneous reaction, the. usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its. In heterogeneous catalysis, catalysts provide a surface. Adding a drop of blood to. catalysts allow a reaction to proceed via a pathway that has a. Catalyst In Chemistry Example.