Chlorine Reaction Redox . this reaction satisfies the criterion for redox classification, since the oxidation number for na is decreased from +1 to 0 (it undergoes. the reaction of chlorine with dilute alkali is an example of a disproportionation reaction. In the process, the chlorine is reduced to chloride ions. learn what a redox reaction is and how to identify and balance redox reactions. Chlorine gas oxidises iron (ii) ions to iron (iii) ions. In these reactions, the chlorine gets oxidised. oxidation and reduction of chlorine produces different inorganic species, including the chlorine oxyanions hypochlorite (clo −). some redox reactions involve the transfer of electrons between reactant species to yield ionic products, such as the reaction between sodium and chlorine to yield sodium. Explore oxidation, reduction, and oxidation numbers. The reaction between chlorine and iron (ii) ions. oxidation state is a number assigned to an element in a compound according to some rules.
from www.numerade.com
oxidation and reduction of chlorine produces different inorganic species, including the chlorine oxyanions hypochlorite (clo −). learn what a redox reaction is and how to identify and balance redox reactions. Explore oxidation, reduction, and oxidation numbers. Chlorine gas oxidises iron (ii) ions to iron (iii) ions. In these reactions, the chlorine gets oxidised. some redox reactions involve the transfer of electrons between reactant species to yield ionic products, such as the reaction between sodium and chlorine to yield sodium. The reaction between chlorine and iron (ii) ions. oxidation state is a number assigned to an element in a compound according to some rules. the reaction of chlorine with dilute alkali is an example of a disproportionation reaction. In the process, the chlorine is reduced to chloride ions.
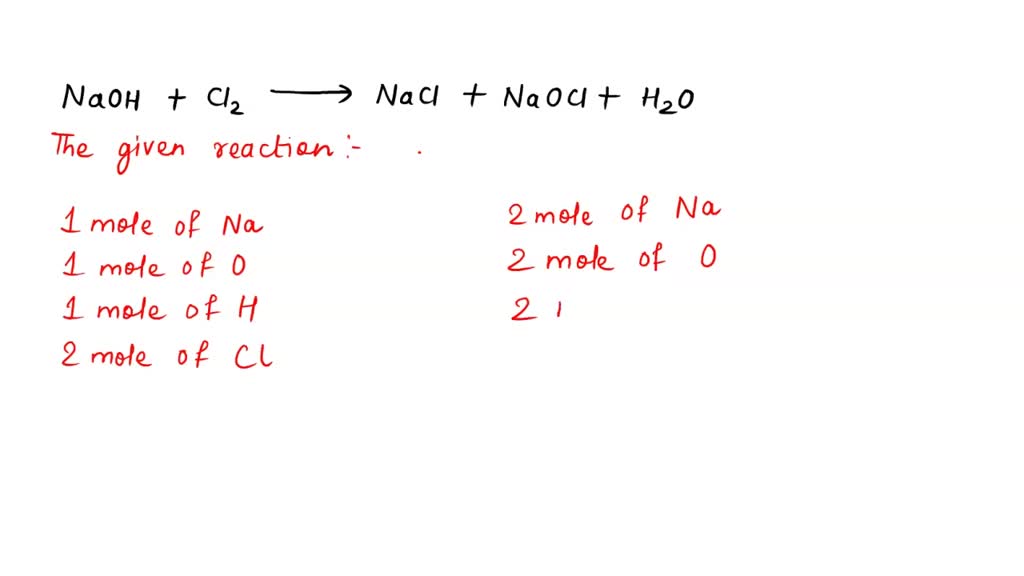
SOLVED 1. Household bleach (NaOCl) is really a solution of sodium
Chlorine Reaction Redox The reaction between chlorine and iron (ii) ions. some redox reactions involve the transfer of electrons between reactant species to yield ionic products, such as the reaction between sodium and chlorine to yield sodium. learn what a redox reaction is and how to identify and balance redox reactions. this reaction satisfies the criterion for redox classification, since the oxidation number for na is decreased from +1 to 0 (it undergoes. the reaction of chlorine with dilute alkali is an example of a disproportionation reaction. In the process, the chlorine is reduced to chloride ions. Explore oxidation, reduction, and oxidation numbers. In these reactions, the chlorine gets oxidised. Chlorine gas oxidises iron (ii) ions to iron (iii) ions. The reaction between chlorine and iron (ii) ions. oxidation and reduction of chlorine produces different inorganic species, including the chlorine oxyanions hypochlorite (clo −). oxidation state is a number assigned to an element in a compound according to some rules.
From www.oxfamwash.org
Chlorination for Water Treatment in Humanitarian Responses Oxfam WASH Chlorine Reaction Redox Explore oxidation, reduction, and oxidation numbers. In the process, the chlorine is reduced to chloride ions. The reaction between chlorine and iron (ii) ions. Chlorine gas oxidises iron (ii) ions to iron (iii) ions. this reaction satisfies the criterion for redox classification, since the oxidation number for na is decreased from +1 to 0 (it undergoes. some redox. Chlorine Reaction Redox.
From www.masterorganicchemistry.com
Reduction of Nitro Groups, The BaeyerVilliger, and Protection of Amines Chlorine Reaction Redox some redox reactions involve the transfer of electrons between reactant species to yield ionic products, such as the reaction between sodium and chlorine to yield sodium. the reaction of chlorine with dilute alkali is an example of a disproportionation reaction. this reaction satisfies the criterion for redox classification, since the oxidation number for na is decreased from. Chlorine Reaction Redox.
From en.ppt-online.org
Uses of chlorine and its compounds online presentation Chlorine Reaction Redox In these reactions, the chlorine gets oxidised. the reaction of chlorine with dilute alkali is an example of a disproportionation reaction. In the process, the chlorine is reduced to chloride ions. learn what a redox reaction is and how to identify and balance redox reactions. The reaction between chlorine and iron (ii) ions. this reaction satisfies the. Chlorine Reaction Redox.
From www.pinterest.nz
Oxidation Reduction (Redox) Reactions Balancing Redox Reactions Chlorine Reaction Redox In these reactions, the chlorine gets oxidised. some redox reactions involve the transfer of electrons between reactant species to yield ionic products, such as the reaction between sodium and chlorine to yield sodium. the reaction of chlorine with dilute alkali is an example of a disproportionation reaction. In the process, the chlorine is reduced to chloride ions. Explore. Chlorine Reaction Redox.
From shaunmwilliams.com
Chapter 4 Presentation Chlorine Reaction Redox The reaction between chlorine and iron (ii) ions. this reaction satisfies the criterion for redox classification, since the oxidation number for na is decreased from +1 to 0 (it undergoes. Explore oxidation, reduction, and oxidation numbers. In the process, the chlorine is reduced to chloride ions. oxidation state is a number assigned to an element in a compound. Chlorine Reaction Redox.
From www.youtube.com
RedOx Writing RedOx HalfReactions YouTube Chlorine Reaction Redox this reaction satisfies the criterion for redox classification, since the oxidation number for na is decreased from +1 to 0 (it undergoes. Explore oxidation, reduction, and oxidation numbers. learn what a redox reaction is and how to identify and balance redox reactions. In these reactions, the chlorine gets oxidised. The reaction between chlorine and iron (ii) ions. In. Chlorine Reaction Redox.
From www.youtube.com
Balance redox reaction in alkaline medium YouTube Chlorine Reaction Redox this reaction satisfies the criterion for redox classification, since the oxidation number for na is decreased from +1 to 0 (it undergoes. oxidation state is a number assigned to an element in a compound according to some rules. In these reactions, the chlorine gets oxidised. learn what a redox reaction is and how to identify and balance. Chlorine Reaction Redox.
From blog.chloramineconsulting.com
Pool Water Chemistry, Part 1 Pool Sanitization Chlorine Reaction Redox the reaction of chlorine with dilute alkali is an example of a disproportionation reaction. In these reactions, the chlorine gets oxidised. oxidation state is a number assigned to an element in a compound according to some rules. this reaction satisfies the criterion for redox classification, since the oxidation number for na is decreased from +1 to 0. Chlorine Reaction Redox.
From www.tessshebaylo.com
Balanced Chemical Equation For Hydrogen Peroxide And Bleach Tessshebaylo Chlorine Reaction Redox the reaction of chlorine with dilute alkali is an example of a disproportionation reaction. some redox reactions involve the transfer of electrons between reactant species to yield ionic products, such as the reaction between sodium and chlorine to yield sodium. Chlorine gas oxidises iron (ii) ions to iron (iii) ions. oxidation state is a number assigned to. Chlorine Reaction Redox.
From www.sarthaks.com
When `Cl_(2)` gas reacts with hot and concentrated sodium hydroxide Chlorine Reaction Redox Chlorine gas oxidises iron (ii) ions to iron (iii) ions. learn what a redox reaction is and how to identify and balance redox reactions. In these reactions, the chlorine gets oxidised. the reaction of chlorine with dilute alkali is an example of a disproportionation reaction. some redox reactions involve the transfer of electrons between reactant species to. Chlorine Reaction Redox.
From www.numerade.com
SOLVED 1. Household bleach (NaOCl) is really a solution of sodium Chlorine Reaction Redox Chlorine gas oxidises iron (ii) ions to iron (iii) ions. In the process, the chlorine is reduced to chloride ions. oxidation state is a number assigned to an element in a compound according to some rules. The reaction between chlorine and iron (ii) ions. the reaction of chlorine with dilute alkali is an example of a disproportionation reaction.. Chlorine Reaction Redox.
From pubs.acs.org
Thiol Chlorination with NChlorosuccinimide HClCatalyzed Release of Chlorine Reaction Redox In these reactions, the chlorine gets oxidised. oxidation and reduction of chlorine produces different inorganic species, including the chlorine oxyanions hypochlorite (clo −). the reaction of chlorine with dilute alkali is an example of a disproportionation reaction. oxidation state is a number assigned to an element in a compound according to some rules. this reaction satisfies. Chlorine Reaction Redox.
From www.youtube.com
Cyanuric chloride Basic idea, preparation and use in organic synthesis Chlorine Reaction Redox oxidation and reduction of chlorine produces different inorganic species, including the chlorine oxyanions hypochlorite (clo −). this reaction satisfies the criterion for redox classification, since the oxidation number for na is decreased from +1 to 0 (it undergoes. Explore oxidation, reduction, and oxidation numbers. oxidation state is a number assigned to an element in a compound according. Chlorine Reaction Redox.
From www.sarthaks.com
When `Cl_(2)` gas reacts with hot and concentrated sodium hydroxide Chlorine Reaction Redox Explore oxidation, reduction, and oxidation numbers. learn what a redox reaction is and how to identify and balance redox reactions. the reaction of chlorine with dilute alkali is an example of a disproportionation reaction. oxidation state is a number assigned to an element in a compound according to some rules. In the process, the chlorine is reduced. Chlorine Reaction Redox.
From fity.club
Chlorine Gas Formula Chemical Formula Of Chlorine Gas On Chlorine Reaction Redox learn what a redox reaction is and how to identify and balance redox reactions. some redox reactions involve the transfer of electrons between reactant species to yield ionic products, such as the reaction between sodium and chlorine to yield sodium. oxidation and reduction of chlorine produces different inorganic species, including the chlorine oxyanions hypochlorite (clo −). The. Chlorine Reaction Redox.
From fphoto.photoshelter.com
science chemistry single replacement redox reaction chlorine Chlorine Reaction Redox oxidation and reduction of chlorine produces different inorganic species, including the chlorine oxyanions hypochlorite (clo −). In the process, the chlorine is reduced to chloride ions. Explore oxidation, reduction, and oxidation numbers. Chlorine gas oxidises iron (ii) ions to iron (iii) ions. learn what a redox reaction is and how to identify and balance redox reactions. In these. Chlorine Reaction Redox.
From www.youtube.com
Type of Reaction for Na + Cl2 = NaCl (Sodium + Chlorine gas) YouTube Chlorine Reaction Redox In the process, the chlorine is reduced to chloride ions. The reaction between chlorine and iron (ii) ions. oxidation state is a number assigned to an element in a compound according to some rules. this reaction satisfies the criterion for redox classification, since the oxidation number for na is decreased from +1 to 0 (it undergoes. Chlorine gas. Chlorine Reaction Redox.
From www.youtube.com
How to Balance P + Cl2 = PCl3 (Phosphorous + Chlorine gas) YouTube Chlorine Reaction Redox In the process, the chlorine is reduced to chloride ions. oxidation and reduction of chlorine produces different inorganic species, including the chlorine oxyanions hypochlorite (clo −). the reaction of chlorine with dilute alkali is an example of a disproportionation reaction. learn what a redox reaction is and how to identify and balance redox reactions. In these reactions,. Chlorine Reaction Redox.
From www.slideserve.com
PPT Reactions of chlorine with water and sodium hydroxide. PowerPoint Chlorine Reaction Redox some redox reactions involve the transfer of electrons between reactant species to yield ionic products, such as the reaction between sodium and chlorine to yield sodium. The reaction between chlorine and iron (ii) ions. Explore oxidation, reduction, and oxidation numbers. In these reactions, the chlorine gets oxidised. this reaction satisfies the criterion for redox classification, since the oxidation. Chlorine Reaction Redox.
From www.mindomo.com
Organic Reactions Mind Map Chlorine Reaction Redox In the process, the chlorine is reduced to chloride ions. oxidation and reduction of chlorine produces different inorganic species, including the chlorine oxyanions hypochlorite (clo −). Explore oxidation, reduction, and oxidation numbers. oxidation state is a number assigned to an element in a compound according to some rules. The reaction between chlorine and iron (ii) ions. this. Chlorine Reaction Redox.
From www.masterorganicchemistry.com
Synthesis (2) Reactions of Alkanes — Master Organic Chemistry Chlorine Reaction Redox the reaction of chlorine with dilute alkali is an example of a disproportionation reaction. Explore oxidation, reduction, and oxidation numbers. The reaction between chlorine and iron (ii) ions. In these reactions, the chlorine gets oxidised. oxidation state is a number assigned to an element in a compound according to some rules. oxidation and reduction of chlorine produces. Chlorine Reaction Redox.
From www.coursehero.com
Solved Chlorination of o xylene ( o dimethylbenzene) yields a Chlorine Reaction Redox Chlorine gas oxidises iron (ii) ions to iron (iii) ions. oxidation state is a number assigned to an element in a compound according to some rules. some redox reactions involve the transfer of electrons between reactant species to yield ionic products, such as the reaction between sodium and chlorine to yield sodium. learn what a redox reaction. Chlorine Reaction Redox.
From www.chemistrystudent.com
Redox (ALevel) ChemistryStudent Chlorine Reaction Redox In the process, the chlorine is reduced to chloride ions. this reaction satisfies the criterion for redox classification, since the oxidation number for na is decreased from +1 to 0 (it undergoes. learn what a redox reaction is and how to identify and balance redox reactions. some redox reactions involve the transfer of electrons between reactant species. Chlorine Reaction Redox.
From owlcation.com
AS Chemistry Redox Reactions and Group 2 Elements Owlcation Chlorine Reaction Redox In these reactions, the chlorine gets oxidised. oxidation state is a number assigned to an element in a compound according to some rules. The reaction between chlorine and iron (ii) ions. this reaction satisfies the criterion for redox classification, since the oxidation number for na is decreased from +1 to 0 (it undergoes. some redox reactions involve. Chlorine Reaction Redox.
From spmchemistry.blog.onlinetuition.com.my
Examples of Redox Reaction Displacement of Halogen SPM Chemistry Chlorine Reaction Redox learn what a redox reaction is and how to identify and balance redox reactions. the reaction of chlorine with dilute alkali is an example of a disproportionation reaction. Chlorine gas oxidises iron (ii) ions to iron (iii) ions. some redox reactions involve the transfer of electrons between reactant species to yield ionic products, such as the reaction. Chlorine Reaction Redox.
From www.dreamstime.com
Redox Reaction Infographic Diagram Stock Vector Illustration of Chlorine Reaction Redox In the process, the chlorine is reduced to chloride ions. Explore oxidation, reduction, and oxidation numbers. In these reactions, the chlorine gets oxidised. Chlorine gas oxidises iron (ii) ions to iron (iii) ions. learn what a redox reaction is and how to identify and balance redox reactions. this reaction satisfies the criterion for redox classification, since the oxidation. Chlorine Reaction Redox.
From www.youtube.com
Chlorine and Water AS Chemistry YouTube Chlorine Reaction Redox some redox reactions involve the transfer of electrons between reactant species to yield ionic products, such as the reaction between sodium and chlorine to yield sodium. oxidation and reduction of chlorine produces different inorganic species, including the chlorine oxyanions hypochlorite (clo −). the reaction of chlorine with dilute alkali is an example of a disproportionation reaction. Chlorine. Chlorine Reaction Redox.
From pubs.rsc.org
439. Chlorine activation by redoxtransfer. Part I. The reaction Chlorine Reaction Redox learn what a redox reaction is and how to identify and balance redox reactions. oxidation and reduction of chlorine produces different inorganic species, including the chlorine oxyanions hypochlorite (clo −). In the process, the chlorine is reduced to chloride ions. this reaction satisfies the criterion for redox classification, since the oxidation number for na is decreased from. Chlorine Reaction Redox.
From www.slideserve.com
PPT Chapter 4 PowerPoint Presentation, free download ID1951818 Chlorine Reaction Redox Explore oxidation, reduction, and oxidation numbers. this reaction satisfies the criterion for redox classification, since the oxidation number for na is decreased from +1 to 0 (it undergoes. In these reactions, the chlorine gets oxidised. some redox reactions involve the transfer of electrons between reactant species to yield ionic products, such as the reaction between sodium and chlorine. Chlorine Reaction Redox.
From exontsuic.blob.core.windows.net
Chlorine Gas Balanced Equation at Thomas Sheehan blog Chlorine Reaction Redox learn what a redox reaction is and how to identify and balance redox reactions. oxidation state is a number assigned to an element in a compound according to some rules. The reaction between chlorine and iron (ii) ions. oxidation and reduction of chlorine produces different inorganic species, including the chlorine oxyanions hypochlorite (clo −). the reaction. Chlorine Reaction Redox.
From byjus.com
Chlorine reacts with hot and concentrated NaOH and produces compounds X Chlorine Reaction Redox oxidation and reduction of chlorine produces different inorganic species, including the chlorine oxyanions hypochlorite (clo −). Chlorine gas oxidises iron (ii) ions to iron (iii) ions. some redox reactions involve the transfer of electrons between reactant species to yield ionic products, such as the reaction between sodium and chlorine to yield sodium. The reaction between chlorine and iron. Chlorine Reaction Redox.
From spmchemistry.blog.onlinetuition.com.my
Reaction of Alkali Metals with Chlorine SPM Chemistry Chlorine Reaction Redox Explore oxidation, reduction, and oxidation numbers. The reaction between chlorine and iron (ii) ions. Chlorine gas oxidises iron (ii) ions to iron (iii) ions. oxidation and reduction of chlorine produces different inorganic species, including the chlorine oxyanions hypochlorite (clo −). oxidation state is a number assigned to an element in a compound according to some rules. some. Chlorine Reaction Redox.
From signalticket9.pythonanywhere.com
Awesome Khan Academy Redox Reactions Chemistry Reaction Calculator Chlorine Reaction Redox Explore oxidation, reduction, and oxidation numbers. The reaction between chlorine and iron (ii) ions. oxidation and reduction of chlorine produces different inorganic species, including the chlorine oxyanions hypochlorite (clo −). this reaction satisfies the criterion for redox classification, since the oxidation number for na is decreased from +1 to 0 (it undergoes. In the process, the chlorine is. Chlorine Reaction Redox.
From www.masterorganicchemistry.com
Thionyl Chloride (SOCl2) Master Organic Chemistry Chlorine Reaction Redox oxidation state is a number assigned to an element in a compound according to some rules. The reaction between chlorine and iron (ii) ions. oxidation and reduction of chlorine produces different inorganic species, including the chlorine oxyanions hypochlorite (clo −). In the process, the chlorine is reduced to chloride ions. Chlorine gas oxidises iron (ii) ions to iron. Chlorine Reaction Redox.
From ar.inspiredpencil.com
Electrophilic Aromatic Substitution Mechanism Chlorination Chlorine Reaction Redox Chlorine gas oxidises iron (ii) ions to iron (iii) ions. this reaction satisfies the criterion for redox classification, since the oxidation number for na is decreased from +1 to 0 (it undergoes. learn what a redox reaction is and how to identify and balance redox reactions. In the process, the chlorine is reduced to chloride ions. some. Chlorine Reaction Redox.