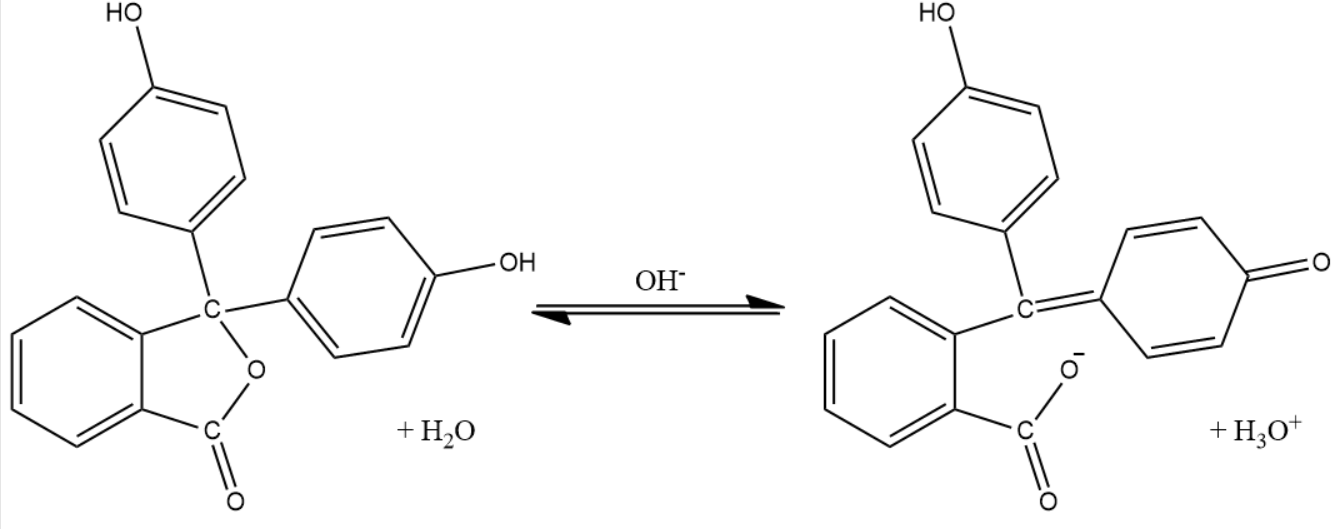
How Does Phenolphthalein Indicator Work . Phenolphthalein is a ph indicator that changes color based on the acidity or alkalinity of a solution. In an acidic solution, phenolphthalein remains colorless, but in an alkaline solution, it turns pink. For example, common indicators such as phenolphthalein, methyl red, and bromothymol blue are used to indicate ph ranges of about 8 to 10, 4.5 to 6, and 6 to 7.5 accordingly. It also serves as a component of universal indicator, together with. It helps determine the ph of a solution. Ph indicators are specific to the range of ph values one wishes to observe. The phenolphthalein indicator is colorless below a ph of 8.5 but turns pink to deep red above a ph of 9.0. Its structure consists of three aromatic rings connected by two carbon atoms.
from proper-cooking.info
For example, common indicators such as phenolphthalein, methyl red, and bromothymol blue are used to indicate ph ranges of about 8 to 10, 4.5 to 6, and 6 to 7.5 accordingly. The phenolphthalein indicator is colorless below a ph of 8.5 but turns pink to deep red above a ph of 9.0. Phenolphthalein is a ph indicator that changes color based on the acidity or alkalinity of a solution. In an acidic solution, phenolphthalein remains colorless, but in an alkaline solution, it turns pink. It also serves as a component of universal indicator, together with. Its structure consists of three aromatic rings connected by two carbon atoms. Ph indicators are specific to the range of ph values one wishes to observe. It helps determine the ph of a solution.
Phenolphthalein Titration
How Does Phenolphthalein Indicator Work Ph indicators are specific to the range of ph values one wishes to observe. Phenolphthalein is a ph indicator that changes color based on the acidity or alkalinity of a solution. Its structure consists of three aromatic rings connected by two carbon atoms. For example, common indicators such as phenolphthalein, methyl red, and bromothymol blue are used to indicate ph ranges of about 8 to 10, 4.5 to 6, and 6 to 7.5 accordingly. In an acidic solution, phenolphthalein remains colorless, but in an alkaline solution, it turns pink. Ph indicators are specific to the range of ph values one wishes to observe. It also serves as a component of universal indicator, together with. The phenolphthalein indicator is colorless below a ph of 8.5 but turns pink to deep red above a ph of 9.0. It helps determine the ph of a solution.
From www.libertysci.com
Phenolphthalein Indicator Solution, 2 ethanolic, 100 mL Liberty How Does Phenolphthalein Indicator Work It helps determine the ph of a solution. Ph indicators are specific to the range of ph values one wishes to observe. The phenolphthalein indicator is colorless below a ph of 8.5 but turns pink to deep red above a ph of 9.0. It also serves as a component of universal indicator, together with. In an acidic solution, phenolphthalein remains. How Does Phenolphthalein Indicator Work.
From demiross.z13.web.core.windows.net
Litmus Paper Color Chart How Does Phenolphthalein Indicator Work Ph indicators are specific to the range of ph values one wishes to observe. Its structure consists of three aromatic rings connected by two carbon atoms. In an acidic solution, phenolphthalein remains colorless, but in an alkaline solution, it turns pink. Phenolphthalein is a ph indicator that changes color based on the acidity or alkalinity of a solution. It helps. How Does Phenolphthalein Indicator Work.
From www.desertcart.ae
Buy pH Indicator Set with Phenolphthalein, Universal Indicator How Does Phenolphthalein Indicator Work Phenolphthalein is a ph indicator that changes color based on the acidity or alkalinity of a solution. Its structure consists of three aromatic rings connected by two carbon atoms. It also serves as a component of universal indicator, together with. In an acidic solution, phenolphthalein remains colorless, but in an alkaline solution, it turns pink. The phenolphthalein indicator is colorless. How Does Phenolphthalein Indicator Work.
From www.sciencephoto.com
Phenolphthalein Indicator Stock Image C039/1215 Science Photo Library How Does Phenolphthalein Indicator Work Phenolphthalein is a ph indicator that changes color based on the acidity or alkalinity of a solution. The phenolphthalein indicator is colorless below a ph of 8.5 but turns pink to deep red above a ph of 9.0. In an acidic solution, phenolphthalein remains colorless, but in an alkaline solution, it turns pink. It helps determine the ph of a. How Does Phenolphthalein Indicator Work.
From www.chegg.com
Solved Colours of phenolphthalein indicator 100 100 cm How Does Phenolphthalein Indicator Work The phenolphthalein indicator is colorless below a ph of 8.5 but turns pink to deep red above a ph of 9.0. It helps determine the ph of a solution. It also serves as a component of universal indicator, together with. Phenolphthalein is a ph indicator that changes color based on the acidity or alkalinity of a solution. For example, common. How Does Phenolphthalein Indicator Work.
From rxmarine.com
Phenolphthalein pH Indicator 1 Solution Manufacturer, Supplier, Exporter How Does Phenolphthalein Indicator Work It helps determine the ph of a solution. For example, common indicators such as phenolphthalein, methyl red, and bromothymol blue are used to indicate ph ranges of about 8 to 10, 4.5 to 6, and 6 to 7.5 accordingly. The phenolphthalein indicator is colorless below a ph of 8.5 but turns pink to deep red above a ph of 9.0.. How Does Phenolphthalein Indicator Work.
From hashimanes.blogspot.com
32+ Why Does Phenolphthalein Turn Pink HashimAnes How Does Phenolphthalein Indicator Work It also serves as a component of universal indicator, together with. For example, common indicators such as phenolphthalein, methyl red, and bromothymol blue are used to indicate ph ranges of about 8 to 10, 4.5 to 6, and 6 to 7.5 accordingly. Its structure consists of three aromatic rings connected by two carbon atoms. In an acidic solution, phenolphthalein remains. How Does Phenolphthalein Indicator Work.
From quizrapturised.z4.web.core.windows.net
What Color Are Acids How Does Phenolphthalein Indicator Work The phenolphthalein indicator is colorless below a ph of 8.5 but turns pink to deep red above a ph of 9.0. It also serves as a component of universal indicator, together with. Ph indicators are specific to the range of ph values one wishes to observe. Phenolphthalein is a ph indicator that changes color based on the acidity or alkalinity. How Does Phenolphthalein Indicator Work.
From www.ubuy.hk
Phenolphthalein pH Indicator Solution 500 mL Hong Kong Ubuy How Does Phenolphthalein Indicator Work It also serves as a component of universal indicator, together with. Phenolphthalein is a ph indicator that changes color based on the acidity or alkalinity of a solution. Its structure consists of three aromatic rings connected by two carbon atoms. For example, common indicators such as phenolphthalein, methyl red, and bromothymol blue are used to indicate ph ranges of about. How Does Phenolphthalein Indicator Work.
From www.indiamart.com
Phenolphthalein 1 Indicator Solution at best price in Mumbai How Does Phenolphthalein Indicator Work Its structure consists of three aromatic rings connected by two carbon atoms. The phenolphthalein indicator is colorless below a ph of 8.5 but turns pink to deep red above a ph of 9.0. Phenolphthalein is a ph indicator that changes color based on the acidity or alkalinity of a solution. Ph indicators are specific to the range of ph values. How Does Phenolphthalein Indicator Work.
From www.myxxgirl.com
Phenolphthalein Ph Scale My XXX Hot Girl How Does Phenolphthalein Indicator Work Its structure consists of three aromatic rings connected by two carbon atoms. Phenolphthalein is a ph indicator that changes color based on the acidity or alkalinity of a solution. In an acidic solution, phenolphthalein remains colorless, but in an alkaline solution, it turns pink. It helps determine the ph of a solution. The phenolphthalein indicator is colorless below a ph. How Does Phenolphthalein Indicator Work.
From www.teachoo.com
MCQ An aqueous solution ‘A’ turns phenolphthalein solution pink. On How Does Phenolphthalein Indicator Work It helps determine the ph of a solution. For example, common indicators such as phenolphthalein, methyl red, and bromothymol blue are used to indicate ph ranges of about 8 to 10, 4.5 to 6, and 6 to 7.5 accordingly. Phenolphthalein is a ph indicator that changes color based on the acidity or alkalinity of a solution. Ph indicators are specific. How Does Phenolphthalein Indicator Work.
From www.indiamart.com
Phenolphthalein Indicator Solution at Rs 110/bottle Mumbai ID How Does Phenolphthalein Indicator Work In an acidic solution, phenolphthalein remains colorless, but in an alkaline solution, it turns pink. Its structure consists of three aromatic rings connected by two carbon atoms. For example, common indicators such as phenolphthalein, methyl red, and bromothymol blue are used to indicate ph ranges of about 8 to 10, 4.5 to 6, and 6 to 7.5 accordingly. It helps. How Does Phenolphthalein Indicator Work.
From proper-cooking.info
Phenolphthalein Titration How Does Phenolphthalein Indicator Work Phenolphthalein is a ph indicator that changes color based on the acidity or alkalinity of a solution. It helps determine the ph of a solution. In an acidic solution, phenolphthalein remains colorless, but in an alkaline solution, it turns pink. The phenolphthalein indicator is colorless below a ph of 8.5 but turns pink to deep red above a ph of. How Does Phenolphthalein Indicator Work.
From ar.inspiredpencil.com
Methyl Orange Indicator How Does Phenolphthalein Indicator Work It also serves as a component of universal indicator, together with. In an acidic solution, phenolphthalein remains colorless, but in an alkaline solution, it turns pink. Ph indicators are specific to the range of ph values one wishes to observe. Phenolphthalein is a ph indicator that changes color based on the acidity or alkalinity of a solution. The phenolphthalein indicator. How Does Phenolphthalein Indicator Work.
From labinsider.vn
Phenolphthalein ( Indicator grade, pure) Labinsider.vn How Does Phenolphthalein Indicator Work Ph indicators are specific to the range of ph values one wishes to observe. In an acidic solution, phenolphthalein remains colorless, but in an alkaline solution, it turns pink. For example, common indicators such as phenolphthalein, methyl red, and bromothymol blue are used to indicate ph ranges of about 8 to 10, 4.5 to 6, and 6 to 7.5 accordingly.. How Does Phenolphthalein Indicator Work.
From www.watsons.ca
Phenolphthalein Indicator Watson's Barrels & Wine Making Supplies How Does Phenolphthalein Indicator Work The phenolphthalein indicator is colorless below a ph of 8.5 but turns pink to deep red above a ph of 9.0. It helps determine the ph of a solution. Its structure consists of three aromatic rings connected by two carbon atoms. Phenolphthalein is a ph indicator that changes color based on the acidity or alkalinity of a solution. For example,. How Does Phenolphthalein Indicator Work.
From www.youtube.com
Making Phenolphthalein (a common pH indicator) YouTube How Does Phenolphthalein Indicator Work In an acidic solution, phenolphthalein remains colorless, but in an alkaline solution, it turns pink. It also serves as a component of universal indicator, together with. Ph indicators are specific to the range of ph values one wishes to observe. It helps determine the ph of a solution. Its structure consists of three aromatic rings connected by two carbon atoms.. How Does Phenolphthalein Indicator Work.
From mungfali.com
Airplane Airspeed Indicator How Does Phenolphthalein Indicator Work Ph indicators are specific to the range of ph values one wishes to observe. Phenolphthalein is a ph indicator that changes color based on the acidity or alkalinity of a solution. It helps determine the ph of a solution. For example, common indicators such as phenolphthalein, methyl red, and bromothymol blue are used to indicate ph ranges of about 8. How Does Phenolphthalein Indicator Work.
From www.entrancei.com
Indicators colours, how Indicators works ? Phenolphthalein Methyl orange. How Does Phenolphthalein Indicator Work It helps determine the ph of a solution. Phenolphthalein is a ph indicator that changes color based on the acidity or alkalinity of a solution. The phenolphthalein indicator is colorless below a ph of 8.5 but turns pink to deep red above a ph of 9.0. Ph indicators are specific to the range of ph values one wishes to observe.. How Does Phenolphthalein Indicator Work.
From hoakhoa.com.vn
Phenolphthalein indicator 1072330100 How Does Phenolphthalein Indicator Work In an acidic solution, phenolphthalein remains colorless, but in an alkaline solution, it turns pink. Phenolphthalein is a ph indicator that changes color based on the acidity or alkalinity of a solution. For example, common indicators such as phenolphthalein, methyl red, and bromothymol blue are used to indicate ph ranges of about 8 to 10, 4.5 to 6, and 6. How Does Phenolphthalein Indicator Work.
From www.indiamart.com
Phenolphthalein Indicator Solution, Drum of 500 ml at Rs 800/bottle in How Does Phenolphthalein Indicator Work Phenolphthalein is a ph indicator that changes color based on the acidity or alkalinity of a solution. In an acidic solution, phenolphthalein remains colorless, but in an alkaline solution, it turns pink. Ph indicators are specific to the range of ph values one wishes to observe. The phenolphthalein indicator is colorless below a ph of 8.5 but turns pink to. How Does Phenolphthalein Indicator Work.
From mavink.com
Phenolphthalein Lewis Structure How Does Phenolphthalein Indicator Work In an acidic solution, phenolphthalein remains colorless, but in an alkaline solution, it turns pink. Its structure consists of three aromatic rings connected by two carbon atoms. For example, common indicators such as phenolphthalein, methyl red, and bromothymol blue are used to indicate ph ranges of about 8 to 10, 4.5 to 6, and 6 to 7.5 accordingly. It also. How Does Phenolphthalein Indicator Work.
From www.researchgate.net
Phenolphthalein test on mortar beams Download Scientific Diagram How Does Phenolphthalein Indicator Work Ph indicators are specific to the range of ph values one wishes to observe. In an acidic solution, phenolphthalein remains colorless, but in an alkaline solution, it turns pink. Its structure consists of three aromatic rings connected by two carbon atoms. Phenolphthalein is a ph indicator that changes color based on the acidity or alkalinity of a solution. The phenolphthalein. How Does Phenolphthalein Indicator Work.
From ar.inspiredpencil.com
Phenolphthalein How Does Phenolphthalein Indicator Work Ph indicators are specific to the range of ph values one wishes to observe. For example, common indicators such as phenolphthalein, methyl red, and bromothymol blue are used to indicate ph ranges of about 8 to 10, 4.5 to 6, and 6 to 7.5 accordingly. The phenolphthalein indicator is colorless below a ph of 8.5 but turns pink to deep. How Does Phenolphthalein Indicator Work.
From mavink.com
Phenolphthalein Ph Range How Does Phenolphthalein Indicator Work For example, common indicators such as phenolphthalein, methyl red, and bromothymol blue are used to indicate ph ranges of about 8 to 10, 4.5 to 6, and 6 to 7.5 accordingly. Ph indicators are specific to the range of ph values one wishes to observe. Its structure consists of three aromatic rings connected by two carbon atoms. Phenolphthalein is a. How Does Phenolphthalein Indicator Work.
From slideplayer.com
How simple indicators work. ppt download How Does Phenolphthalein Indicator Work It helps determine the ph of a solution. It also serves as a component of universal indicator, together with. Its structure consists of three aromatic rings connected by two carbon atoms. Ph indicators are specific to the range of ph values one wishes to observe. In an acidic solution, phenolphthalein remains colorless, but in an alkaline solution, it turns pink.. How Does Phenolphthalein Indicator Work.
From www.sciencephoto.com
Phenolphthalein Indicator Stock Image C030/7311 Science Photo Library How Does Phenolphthalein Indicator Work Its structure consists of three aromatic rings connected by two carbon atoms. Phenolphthalein is a ph indicator that changes color based on the acidity or alkalinity of a solution. It also serves as a component of universal indicator, together with. It helps determine the ph of a solution. The phenolphthalein indicator is colorless below a ph of 8.5 but turns. How Does Phenolphthalein Indicator Work.
From www.homesciencetools.com
Phenolphthalein Solution, 30 ml Home Science Tools How Does Phenolphthalein Indicator Work Phenolphthalein is a ph indicator that changes color based on the acidity or alkalinity of a solution. It helps determine the ph of a solution. It also serves as a component of universal indicator, together with. Its structure consists of three aromatic rings connected by two carbon atoms. The phenolphthalein indicator is colorless below a ph of 8.5 but turns. How Does Phenolphthalein Indicator Work.
From www.numerade.com
SOLVED Why are phenolphthalein indicators used in the nylon synthesis How Does Phenolphthalein Indicator Work For example, common indicators such as phenolphthalein, methyl red, and bromothymol blue are used to indicate ph ranges of about 8 to 10, 4.5 to 6, and 6 to 7.5 accordingly. Phenolphthalein is a ph indicator that changes color based on the acidity or alkalinity of a solution. It helps determine the ph of a solution. Its structure consists of. How Does Phenolphthalein Indicator Work.
From sciencenotes.org
Phenolphthalein Indicator How Does Phenolphthalein Indicator Work It helps determine the ph of a solution. Its structure consists of three aromatic rings connected by two carbon atoms. In an acidic solution, phenolphthalein remains colorless, but in an alkaline solution, it turns pink. Ph indicators are specific to the range of ph values one wishes to observe. Phenolphthalein is a ph indicator that changes color based on the. How Does Phenolphthalein Indicator Work.
From www.entrancei.com
Indicators colours, how Indicators works ? Phenolphthalein Methyl orange. How Does Phenolphthalein Indicator Work In an acidic solution, phenolphthalein remains colorless, but in an alkaline solution, it turns pink. It also serves as a component of universal indicator, together with. Phenolphthalein is a ph indicator that changes color based on the acidity or alkalinity of a solution. The phenolphthalein indicator is colorless below a ph of 8.5 but turns pink to deep red above. How Does Phenolphthalein Indicator Work.
From www.youtube.com
How to make 1 Phenolphthalein Indicator YouTube How Does Phenolphthalein Indicator Work The phenolphthalein indicator is colorless below a ph of 8.5 but turns pink to deep red above a ph of 9.0. It also serves as a component of universal indicator, together with. Its structure consists of three aromatic rings connected by two carbon atoms. For example, common indicators such as phenolphthalein, methyl red, and bromothymol blue are used to indicate. How Does Phenolphthalein Indicator Work.
From ar.inspiredpencil.com
Titration Phenolphthalein How Does Phenolphthalein Indicator Work In an acidic solution, phenolphthalein remains colorless, but in an alkaline solution, it turns pink. For example, common indicators such as phenolphthalein, methyl red, and bromothymol blue are used to indicate ph ranges of about 8 to 10, 4.5 to 6, and 6 to 7.5 accordingly. Phenolphthalein is a ph indicator that changes color based on the acidity or alkalinity. How Does Phenolphthalein Indicator Work.
From www.nagwa.com
Question Video Determining the Color of the Indicator Phenolphthalein How Does Phenolphthalein Indicator Work Its structure consists of three aromatic rings connected by two carbon atoms. For example, common indicators such as phenolphthalein, methyl red, and bromothymol blue are used to indicate ph ranges of about 8 to 10, 4.5 to 6, and 6 to 7.5 accordingly. The phenolphthalein indicator is colorless below a ph of 8.5 but turns pink to deep red above. How Does Phenolphthalein Indicator Work.