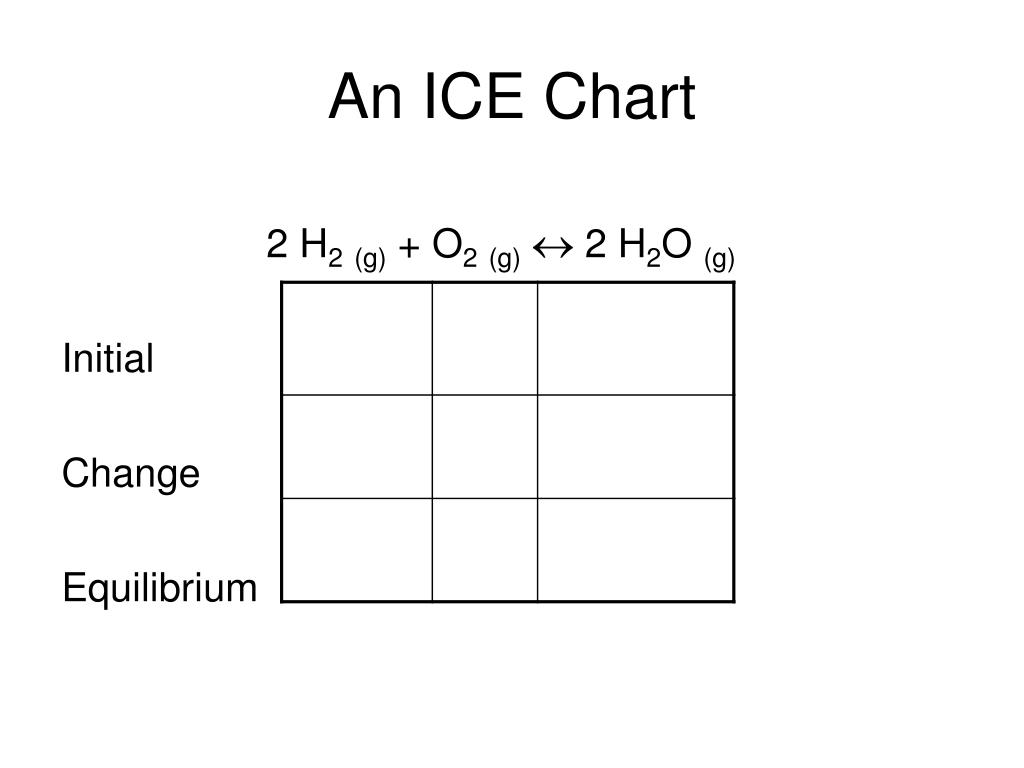
How To Use An Ice Chart . Express all quantities in terms of molarity (moles per liter). Any stoichiometry problem can be solved by following the same. How to make an ice chart. An ice table (acronym for initial concentration, change in concentration, and equilibrium concentration) is an useful methodology for calculating an equilibrium constant from. Ice tables automatically set up and organize the variables and constants needed when calculating the unknown. An ice chart is a tool used in chemistry to keep track of the changes in concentration for each species in a chemical reaction. Ensure the chemical equation for the reaction is balanced. Ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and reacted concentrations. An ice table is a simple organizational tool to solve stoichiometry problems. (if using k p, gaseous species. Ice is a simple acronym for the. Steps for using ice tables. In making an ice chart the following items should be noted:
from www.slideserve.com
An ice chart is a tool used in chemistry to keep track of the changes in concentration for each species in a chemical reaction. Ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and reacted concentrations. Ensure the chemical equation for the reaction is balanced. Ice is a simple acronym for the. Steps for using ice tables. How to make an ice chart. An ice table (acronym for initial concentration, change in concentration, and equilibrium concentration) is an useful methodology for calculating an equilibrium constant from. Ice tables automatically set up and organize the variables and constants needed when calculating the unknown. An ice table is a simple organizational tool to solve stoichiometry problems. (if using k p, gaseous species.
PPT Review of Basic Equilibrium PowerPoint Presentation, free
How To Use An Ice Chart How to make an ice chart. Steps for using ice tables. In making an ice chart the following items should be noted: Express all quantities in terms of molarity (moles per liter). An ice table is a simple organizational tool to solve stoichiometry problems. Ice is a simple acronym for the. Ensure the chemical equation for the reaction is balanced. Ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and reacted concentrations. Any stoichiometry problem can be solved by following the same. An ice table (acronym for initial concentration, change in concentration, and equilibrium concentration) is an useful methodology for calculating an equilibrium constant from. Ice tables automatically set up and organize the variables and constants needed when calculating the unknown. How to make an ice chart. (if using k p, gaseous species. An ice chart is a tool used in chemistry to keep track of the changes in concentration for each species in a chemical reaction.
From solovelytogether.blogspot.com
How To Do Ice Tables Chemistry Decoration Drawing How To Use An Ice Chart An ice chart is a tool used in chemistry to keep track of the changes in concentration for each species in a chemical reaction. How to make an ice chart. In making an ice chart the following items should be noted: Ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and reacted. How To Use An Ice Chart.
From www.youtube.com
ICE Charts Video 2 YouTube How To Use An Ice Chart Ice is a simple acronym for the. An ice table is a simple organizational tool to solve stoichiometry problems. Any stoichiometry problem can be solved by following the same. In making an ice chart the following items should be noted: Ensure the chemical equation for the reaction is balanced. An ice table (acronym for initial concentration, change in concentration, and. How To Use An Ice Chart.
From www.youtube.com
Chemical Equilibrium ICE Table Example 3 YouTube How To Use An Ice Chart Express all quantities in terms of molarity (moles per liter). In making an ice chart the following items should be noted: (if using k p, gaseous species. Ice is a simple acronym for the. How to make an ice chart. An ice table is a simple organizational tool to solve stoichiometry problems. An ice table (acronym for initial concentration, change. How To Use An Ice Chart.
From www.rechargecolorado.org
How To Use Ice Chart Chemistry Best Picture Of Chart How To Use An Ice Chart Express all quantities in terms of molarity (moles per liter). (if using k p, gaseous species. Ice tables automatically set up and organize the variables and constants needed when calculating the unknown. Ensure the chemical equation for the reaction is balanced. How to make an ice chart. Steps for using ice tables. Ice table is used for determining the equilibrium. How To Use An Ice Chart.
From www.youtube.com
14.5 ICE Table Examples and Approximations YouTube How To Use An Ice Chart An ice table is a simple organizational tool to solve stoichiometry problems. How to make an ice chart. Ice is a simple acronym for the. (if using k p, gaseous species. An ice chart is a tool used in chemistry to keep track of the changes in concentration for each species in a chemical reaction. Ice table is used for. How To Use An Ice Chart.
From github.com
GitHub shrinus/ICEchart program that computes ice charts in How To Use An Ice Chart Any stoichiometry problem can be solved by following the same. Express all quantities in terms of molarity (moles per liter). How to make an ice chart. Ice is a simple acronym for the. Ensure the chemical equation for the reaction is balanced. An ice table is a simple organizational tool to solve stoichiometry problems. In making an ice chart the. How To Use An Ice Chart.
From cryo.met.no
Understanding the ice charts cryo.met.no How To Use An Ice Chart Express all quantities in terms of molarity (moles per liter). Ensure the chemical equation for the reaction is balanced. An ice chart is a tool used in chemistry to keep track of the changes in concentration for each species in a chemical reaction. (if using k p, gaseous species. Any stoichiometry problem can be solved by following the same. An. How To Use An Ice Chart.
From solovelytogether.blogspot.com
How To Do Ice Tables Chemistry Decoration Drawing How To Use An Ice Chart Ice is a simple acronym for the. How to make an ice chart. Ice tables automatically set up and organize the variables and constants needed when calculating the unknown. An ice table (acronym for initial concentration, change in concentration, and equilibrium concentration) is an useful methodology for calculating an equilibrium constant from. An ice chart is a tool used in. How To Use An Ice Chart.
From www.pinterest.com
Six Types of Ice, One Guide to Sort Them All Types of ice, Ice cube How To Use An Ice Chart An ice table (acronym for initial concentration, change in concentration, and equilibrium concentration) is an useful methodology for calculating an equilibrium constant from. Ensure the chemical equation for the reaction is balanced. (if using k p, gaseous species. Ice is a simple acronym for the. Express all quantities in terms of molarity (moles per liter). Ice table is used for. How To Use An Ice Chart.
From www.youtube.com
Chemical equilibrium ICE charts YouTube How To Use An Ice Chart Ensure the chemical equation for the reaction is balanced. An ice chart is a tool used in chemistry to keep track of the changes in concentration for each species in a chemical reaction. Ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and reacted concentrations. In making an ice chart the following. How To Use An Ice Chart.
From fishnetics.com
Ice Making Chart How To Use An Ice Chart Ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and reacted concentrations. An ice table (acronym for initial concentration, change in concentration, and equilibrium concentration) is an useful methodology for calculating an equilibrium constant from. Steps for using ice tables. Ice is a simple acronym for the. An ice chart is a. How To Use An Ice Chart.
From www.slideserve.com
PPT Review of Basic Equilibrium PowerPoint Presentation, free How To Use An Ice Chart Ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and reacted concentrations. Any stoichiometry problem can be solved by following the same. Ensure the chemical equation for the reaction is balanced. Steps for using ice tables. Ice is a simple acronym for the. An ice table (acronym for initial concentration, change in. How To Use An Ice Chart.
From www.epa.gov
Climate Change Indicators Ice Sheets US EPA How To Use An Ice Chart An ice chart is a tool used in chemistry to keep track of the changes in concentration for each species in a chemical reaction. (if using k p, gaseous species. How to make an ice chart. Express all quantities in terms of molarity (moles per liter). Ice is a simple acronym for the. In making an ice chart the following. How To Use An Ice Chart.
From www.researchgate.net
Example of weekly ice chart for the western Canadian Arctic produced by How To Use An Ice Chart Steps for using ice tables. Ice tables automatically set up and organize the variables and constants needed when calculating the unknown. An ice table (acronym for initial concentration, change in concentration, and equilibrium concentration) is an useful methodology for calculating an equilibrium constant from. An ice table is a simple organizational tool to solve stoichiometry problems. Ice is a simple. How To Use An Ice Chart.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID How To Use An Ice Chart How to make an ice chart. Any stoichiometry problem can be solved by following the same. Ice is a simple acronym for the. Steps for using ice tables. An ice chart is a tool used in chemistry to keep track of the changes in concentration for each species in a chemical reaction. Ensure the chemical equation for the reaction is. How To Use An Ice Chart.
From www.youtube.com
ICE chart (table) basics YouTube How To Use An Ice Chart Ice tables automatically set up and organize the variables and constants needed when calculating the unknown. An ice chart is a tool used in chemistry to keep track of the changes in concentration for each species in a chemical reaction. Steps for using ice tables. Ensure the chemical equation for the reaction is balanced. Any stoichiometry problem can be solved. How To Use An Ice Chart.
From solovelytogether.blogspot.com
How To Do Ice Tables Chemistry Decoration Drawing How To Use An Ice Chart An ice chart is a tool used in chemistry to keep track of the changes in concentration for each species in a chemical reaction. An ice table is a simple organizational tool to solve stoichiometry problems. Express all quantities in terms of molarity (moles per liter). Ice table is used for determining the equilibrium concentrations based on the reaction quotient. How To Use An Ice Chart.
From davinasswimhouse.com
Davina's Swim House Ice Safety Guide How To Use An Ice Chart Ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and reacted concentrations. Ensure the chemical equation for the reaction is balanced. In making an ice chart the following items should be noted: Steps for using ice tables. An ice table (acronym for initial concentration, change in concentration, and equilibrium concentration) is an. How To Use An Ice Chart.
From cryo.met.no
Understanding the ice charts cryo.met.no How To Use An Ice Chart Ice is a simple acronym for the. Ensure the chemical equation for the reaction is balanced. An ice table is a simple organizational tool to solve stoichiometry problems. Ice tables automatically set up and organize the variables and constants needed when calculating the unknown. In making an ice chart the following items should be noted: Any stoichiometry problem can be. How To Use An Ice Chart.
From cryo.met.no
Understanding the ice charts cryo.met.no How To Use An Ice Chart Ice tables automatically set up and organize the variables and constants needed when calculating the unknown. Ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and reacted concentrations. (if using k p, gaseous species. Any stoichiometry problem can be solved by following the same. How to make an ice chart. Express all. How To Use An Ice Chart.
From sentinel.esa.int
Ice chart of Eastern Greenland Content How To Use An Ice Chart Express all quantities in terms of molarity (moles per liter). Ice is a simple acronym for the. Ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and reacted concentrations. Ice tables automatically set up and organize the variables and constants needed when calculating the unknown. Steps for using ice tables. An ice. How To Use An Ice Chart.
From www.youtube.com
InitialChangeEquilibrium (ICE) Tables YouTube How To Use An Ice Chart An ice table is a simple organizational tool to solve stoichiometry problems. Steps for using ice tables. Ensure the chemical equation for the reaction is balanced. An ice table (acronym for initial concentration, change in concentration, and equilibrium concentration) is an useful methodology for calculating an equilibrium constant from. Ice table is used for determining the equilibrium concentrations based on. How To Use An Ice Chart.
From elchoroukhost.net
Ice Table Calculator Online Elcho Table How To Use An Ice Chart How to make an ice chart. Ice is a simple acronym for the. Ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and reacted concentrations. Steps for using ice tables. An ice table is a simple organizational tool to solve stoichiometry problems. In making an ice chart the following items should be. How To Use An Ice Chart.
From www.slideserve.com
PPT Solution Equilibria PowerPoint Presentation, free download ID How To Use An Ice Chart An ice table is a simple organizational tool to solve stoichiometry problems. In making an ice chart the following items should be noted: (if using k p, gaseous species. Ensure the chemical equation for the reaction is balanced. Ice tables automatically set up and organize the variables and constants needed when calculating the unknown. Ice is a simple acronym for. How To Use An Ice Chart.
From www.slideserve.com
PPT Review of Basic Equilibrium PowerPoint Presentation, free How To Use An Ice Chart Ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and reacted concentrations. An ice chart is a tool used in chemistry to keep track of the changes in concentration for each species in a chemical reaction. Ensure the chemical equation for the reaction is balanced. How to make an ice chart. Any. How To Use An Ice Chart.
From www.slideserve.com
PPT Ice Chart Colour Standard Proposal PowerPoint Presentation, free How To Use An Ice Chart In making an ice chart the following items should be noted: How to make an ice chart. An ice table (acronym for initial concentration, change in concentration, and equilibrium concentration) is an useful methodology for calculating an equilibrium constant from. Ice is a simple acronym for the. Any stoichiometry problem can be solved by following the same. An ice table. How To Use An Ice Chart.
From www.youtube.com
Week 9 9. pH of a buffer using an ICE chart YouTube How To Use An Ice Chart In making an ice chart the following items should be noted: An ice table (acronym for initial concentration, change in concentration, and equilibrium concentration) is an useful methodology for calculating an equilibrium constant from. How to make an ice chart. Steps for using ice tables. Ice is a simple acronym for the. (if using k p, gaseous species. Ice tables. How To Use An Ice Chart.
From solovelytogether.blogspot.com
How To Do Ice Tables Chemistry Decoration Drawing How To Use An Ice Chart An ice table (acronym for initial concentration, change in concentration, and equilibrium concentration) is an useful methodology for calculating an equilibrium constant from. Ensure the chemical equation for the reaction is balanced. An ice table is a simple organizational tool to solve stoichiometry problems. How to make an ice chart. An ice chart is a tool used in chemistry to. How To Use An Ice Chart.
From slidetodoc.com
Ice Chart Colour Codes International Ice Chart Working How To Use An Ice Chart Ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and reacted concentrations. An ice chart is a tool used in chemistry to keep track of the changes in concentration for each species in a chemical reaction. Ice is a simple acronym for the. Express all quantities in terms of molarity (moles per. How To Use An Ice Chart.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID How To Use An Ice Chart In making an ice chart the following items should be noted: Ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and reacted concentrations. Ensure the chemical equation for the reaction is balanced. Ice tables automatically set up and organize the variables and constants needed when calculating the unknown. An ice table is. How To Use An Ice Chart.
From www.tamutom.com
Ice Types and How They Are Made 1 How To Use An Ice Chart Ice tables automatically set up and organize the variables and constants needed when calculating the unknown. Ice is a simple acronym for the. In making an ice chart the following items should be noted: An ice table is a simple organizational tool to solve stoichiometry problems. An ice table (acronym for initial concentration, change in concentration, and equilibrium concentration) is. How To Use An Ice Chart.
From www.researchgate.net
Figure B29 Range of Ice Numerals calculated from CIS ice charts for How To Use An Ice Chart Ice tables automatically set up and organize the variables and constants needed when calculating the unknown. Ice is a simple acronym for the. In making an ice chart the following items should be noted: Any stoichiometry problem can be solved by following the same. An ice chart is a tool used in chemistry to keep track of the changes in. How To Use An Ice Chart.
From www.canadianyachting.ca
Ice Safety Chart is a Helpful Guideline as we Move in Canada’s Winter How To Use An Ice Chart Any stoichiometry problem can be solved by following the same. Express all quantities in terms of molarity (moles per liter). An ice table is a simple organizational tool to solve stoichiometry problems. Ensure the chemical equation for the reaction is balanced. Ice is a simple acronym for the. Ice table is used for determining the equilibrium concentrations based on the. How To Use An Ice Chart.
From www.slideserve.com
PPT Ice Chart Colour Standard Proposal PowerPoint Presentation, free How To Use An Ice Chart Ice tables automatically set up and organize the variables and constants needed when calculating the unknown. An ice chart is a tool used in chemistry to keep track of the changes in concentration for each species in a chemical reaction. An ice table is a simple organizational tool to solve stoichiometry problems. How to make an ice chart. Express all. How To Use An Ice Chart.
From www.slideserve.com
PPT Unit 4 Equilibrium PowerPoint Presentation, free download ID How To Use An Ice Chart An ice chart is a tool used in chemistry to keep track of the changes in concentration for each species in a chemical reaction. Steps for using ice tables. Ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and reacted concentrations. Express all quantities in terms of molarity (moles per liter). In. How To Use An Ice Chart.