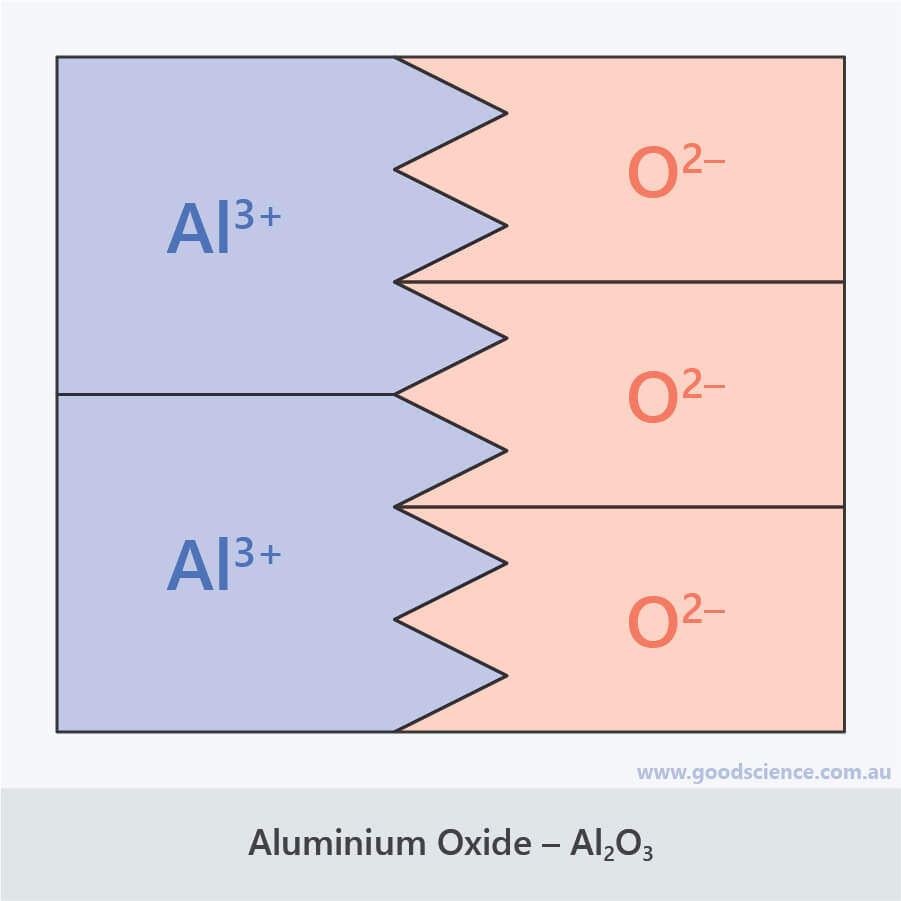
Aluminum Oxide Ionic Formula . The correct answer is #al_2o_3#. Aluminum oxide, also known as alumina, is an inorganic compound composed of aluminum and oxygen. Look at the electronic arrangement of al and o atoms. Aluminium oxide (al 2 o 3 ) uses. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. Aluminium oxide is one of the common ingredients in sunscreen and is also. Aluminium oxide reacts with hot dilute hydrochloric acid to give aluminium chloride solution. Let us see how we got the answer; Aluminum oxide has a chemical formula al2o3. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. It is represented by the chemical. A video explanation of how to write the chemical formula for aluminum oxide. It is amphoteric in nature, and is used in various chemical, industrial and commercial applications. It is considered an indirect additive. Aluminum oxide, with the chemical formula \ (al_2o_3\), is an amphoteric oxide and is commonly referred to as alumina.
from www.goodscience.com.au
The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. The correct answer is #al_2o_3#. Aluminum oxide has a chemical formula al2o3. Look at the electronic arrangement of al and o atoms. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. It is amphoteric in nature, and is used in various chemical, industrial and commercial applications. Aluminum oxide, with the chemical formula \ (al_2o_3\), is an amphoteric oxide and is commonly referred to as alumina. Let us see how we got the answer; A video explanation of how to write the chemical formula for aluminum oxide. Aluminum oxide, also known as alumina, is an inorganic compound composed of aluminum and oxygen.
Determining the Formula for Ionic Compounds Good Science
Aluminum Oxide Ionic Formula It is amphoteric in nature, and is used in various chemical, industrial and commercial applications. Aluminum oxide, also known as alumina, is an inorganic compound composed of aluminum and oxygen. The correct answer is #al_2o_3#. Aluminium oxide is one of the common ingredients in sunscreen and is also. It is represented by the chemical. Let us see how we got the answer; A video explanation of how to write the chemical formula for aluminum oxide. Aluminum oxide has a chemical formula al2o3. It is considered an indirect additive. Aluminum oxide, with the chemical formula \ (al_2o_3\), is an amphoteric oxide and is commonly referred to as alumina. It is amphoteric in nature, and is used in various chemical, industrial and commercial applications. Aluminium oxide reacts with hot dilute hydrochloric acid to give aluminium chloride solution. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. Aluminium oxide (al 2 o 3 ) uses. Look at the electronic arrangement of al and o atoms.
From www.youtube.com
Write the chemical formula of Aluminium oxide YouTube Aluminum Oxide Ionic Formula Look at the electronic arrangement of al and o atoms. Aluminum oxide, also known as alumina, is an inorganic compound composed of aluminum and oxygen. Aluminium oxide reacts with hot dilute hydrochloric acid to give aluminium chloride solution. Aluminum oxide, with the chemical formula \ (al_2o_3\), is an amphoteric oxide and is commonly referred to as alumina. Aluminum oxide has. Aluminum Oxide Ionic Formula.
From molekula.com
Purchase Aluminium oxide basic [1344281] online • Catalog • Molekula Aluminum Oxide Ionic Formula Aluminium oxide is one of the common ingredients in sunscreen and is also. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. The correct answer is #al_2o_3#. A video explanation of how to write the chemical formula for aluminum oxide. Aluminum oxide has a chemical formula al2o3. It is considered. Aluminum Oxide Ionic Formula.
From aluminiumoxidekirikaza.blogspot.com
Aluminium Oxide Aluminium Oxide Ionic Or Covalent Aluminum Oxide Ionic Formula Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. Aluminum oxide, also known as alumina, is an inorganic compound composed of aluminum and oxygen. Aluminium oxide is one of the common ingredients in sunscreen and is also. It is represented by the chemical. Aluminium oxide reacts with hot dilute hydrochloric. Aluminum Oxide Ionic Formula.
From ar.inspiredpencil.com
Aluminum Oxide Lewis Structure Aluminum Oxide Ionic Formula A video explanation of how to write the chemical formula for aluminum oxide. It is amphoteric in nature, and is used in various chemical, industrial and commercial applications. Aluminum oxide has a chemical formula al2o3. Let us see how we got the answer; Look at the electronic arrangement of al and o atoms. The correct answer is #al_2o_3#. Aluminium oxide. Aluminum Oxide Ionic Formula.
From www.slideserve.com
PPT IV. Chemical Bonding PowerPoint Presentation, free download ID Aluminum Oxide Ionic Formula Look at the electronic arrangement of al and o atoms. Aluminum oxide, with the chemical formula \ (al_2o_3\), is an amphoteric oxide and is commonly referred to as alumina. Aluminium oxide reacts with hot dilute hydrochloric acid to give aluminium chloride solution. It is considered an indirect additive. It is represented by the chemical. Let us see how we got. Aluminum Oxide Ionic Formula.
From www.goodscience.com.au
Determining the Formula for Ionic Compounds Good Science Aluminum Oxide Ionic Formula Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. Aluminium oxide reacts with hot dilute hydrochloric acid to give aluminium chloride solution. Let us see how we got the answer; A video explanation of how to write the chemical formula for aluminum oxide. Aluminium oxide is one of the common. Aluminum Oxide Ionic Formula.
From chemistry291.blogspot.com
Formula for Aluminum OxideWhat is the Chemical Formula for Aluminum Aluminum Oxide Ionic Formula Aluminum oxide, with the chemical formula \ (al_2o_3\), is an amphoteric oxide and is commonly referred to as alumina. Aluminium oxide reacts with hot dilute hydrochloric acid to give aluminium chloride solution. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. Aluminium oxide (al 2 o 3 ). Aluminum Oxide Ionic Formula.
From www.slideserve.com
PPT Ionic Compounds PowerPoint Presentation, free download ID3730496 Aluminum Oxide Ionic Formula Aluminium oxide is one of the common ingredients in sunscreen and is also. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. A video explanation of how to write the chemical formula for aluminum oxide. The correct answer is #al_2o_3#. Aluminium oxide reacts with hot dilute hydrochloric acid to give. Aluminum Oxide Ionic Formula.
From www.slideserve.com
PPT Ionic Compounds PowerPoint Presentation, free download ID3730496 Aluminum Oxide Ionic Formula Aluminum oxide has a chemical formula al2o3. It is represented by the chemical. A video explanation of how to write the chemical formula for aluminum oxide. Aluminium oxide (al 2 o 3 ) uses. Aluminum oxide, also known as alumina, is an inorganic compound composed of aluminum and oxygen. Aluminum oxide, with the chemical formula \ (al_2o_3\), is an amphoteric. Aluminum Oxide Ionic Formula.
From www.museoinclusivo.com
Exploring the Chemical Formula of Aluminum Oxide Aluminum Profile Blog Aluminum Oxide Ionic Formula Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. Aluminum oxide, with the chemical formula \ (al_2o_3\), is an amphoteric oxide and is commonly referred to as alumina. Aluminum oxide has a chemical formula al2o3. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose. Aluminum Oxide Ionic Formula.
From www.slideshare.net
Ionic bonding Aluminum Oxide Ionic Formula Aluminium oxide is one of the common ingredients in sunscreen and is also. Aluminium oxide (al 2 o 3 ) uses. Aluminum oxide, also known as alumina, is an inorganic compound composed of aluminum and oxygen. Aluminum oxide has a chemical formula al2o3. Look at the electronic arrangement of al and o atoms. The correct answer is #al_2o_3#. It is. Aluminum Oxide Ionic Formula.
From www.slideserve.com
PPT WarmUp PowerPoint Presentation, free download ID3731178 Aluminum Oxide Ionic Formula Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. Aluminium oxide is one of the common ingredients in sunscreen and is also. The correct answer is #al_2o_3#. Aluminum oxide, with the chemical formula \ (al_2o_3\), is an amphoteric oxide and is commonly referred to as alumina. Let us see how. Aluminum Oxide Ionic Formula.
From www.pw.live
Aluminium Oxide Formula, Structure, Properties, Uses Aluminum Oxide Ionic Formula Aluminum oxide has a chemical formula al2o3. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. Let us see how we got the answer; A video explanation of how to write the chemical formula for aluminum oxide. Aluminium oxide (al 2 o 3 ) uses. The correct answer is #al_2o_3#.. Aluminum Oxide Ionic Formula.
From docslib.org
Ionic Formula Name of Ionic Compound Al2o3 Aluminum Oxide DocsLib Aluminum Oxide Ionic Formula Aluminium oxide is one of the common ingredients in sunscreen and is also. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. Aluminium oxide (al 2 o 3 ) uses. The correct answer is #al_2o_3#. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose. Aluminum Oxide Ionic Formula.
From www.hanlin.com
CIE A Level Chemistry复习笔记1.3.5 Ionic Bonding Examples翰林国际教育 Aluminum Oxide Ionic Formula It is represented by the chemical. Aluminum oxide, also known as alumina, is an inorganic compound composed of aluminum and oxygen. Aluminum oxide has a chemical formula al2o3. Aluminum oxide, with the chemical formula \ (al_2o_3\), is an amphoteric oxide and is commonly referred to as alumina. Aluminium oxide contains oxide ions, and thus reacts with acids in the same. Aluminum Oxide Ionic Formula.
From www.museoinclusivo.com
Exploring the Chemical Formula of Aluminum Oxide Aluminum Profile Blog Aluminum Oxide Ionic Formula It is represented by the chemical. It is amphoteric in nature, and is used in various chemical, industrial and commercial applications. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. Aluminium oxide is one of the common ingredients in sunscreen and is also. Aluminium oxide (al 2 o. Aluminum Oxide Ionic Formula.
From aluminumgenjin.blogspot.com
Aluminum Formula Unit Of Aluminum Oxide Aluminum Oxide Ionic Formula The correct answer is #al_2o_3#. Aluminium oxide is one of the common ingredients in sunscreen and is also. It is amphoteric in nature, and is used in various chemical, industrial and commercial applications. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. Look at the electronic arrangement of. Aluminum Oxide Ionic Formula.
From www.youtube.com
How to write Molecular formula of Aluminium oxide Chemical formula of Aluminum Oxide Ionic Formula The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. A video explanation of how to write the chemical formula for aluminum oxide. Aluminium oxide is one of the common ingredients in sunscreen and is also. Let us see how we got the answer; The correct answer is #al_2o_3#.. Aluminum Oxide Ionic Formula.
From testbook.com
Aluminium Oxide Formula Concept, Structure, Properties and Uses. Aluminum Oxide Ionic Formula It is considered an indirect additive. Aluminum oxide, with the chemical formula \ (al_2o_3\), is an amphoteric oxide and is commonly referred to as alumina. Aluminium oxide reacts with hot dilute hydrochloric acid to give aluminium chloride solution. A video explanation of how to write the chemical formula for aluminum oxide. Aluminium oxide contains oxide ions, and thus reacts with. Aluminum Oxide Ionic Formula.
From www.slideserve.com
PPT Ionic Nomenclature PowerPoint Presentation, free download ID Aluminum Oxide Ionic Formula Look at the electronic arrangement of al and o atoms. A video explanation of how to write the chemical formula for aluminum oxide. It is considered an indirect additive. Aluminium oxide (al 2 o 3 ) uses. Aluminium oxide is one of the common ingredients in sunscreen and is also. The lighter group 3a metals (aluminum, galium and indium), along. Aluminum Oxide Ionic Formula.
From www.museoinclusivo.com
Exploring the Formula for Aluminum Oxide Aluminum Profile Blog Aluminum Oxide Ionic Formula The correct answer is #al_2o_3#. Aluminium oxide (al 2 o 3 ) uses. Aluminium oxide is one of the common ingredients in sunscreen and is also. Aluminium oxide reacts with hot dilute hydrochloric acid to give aluminium chloride solution. It is considered an indirect additive. Aluminum oxide has a chemical formula al2o3. Let us see how we got the answer;. Aluminum Oxide Ionic Formula.
From ar.inspiredpencil.com
Aluminum Oxide Structure Aluminum Oxide Ionic Formula Aluminium oxide reacts with hot dilute hydrochloric acid to give aluminium chloride solution. Aluminum oxide has a chemical formula al2o3. It is represented by the chemical. The correct answer is #al_2o_3#. Aluminium oxide (al 2 o 3 ) uses. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. Aluminium oxide. Aluminum Oxide Ionic Formula.
From www.youtube.com
Is Al2O3 (Aluminum oxide) Ionic or Covalent? YouTube Aluminum Oxide Ionic Formula Look at the electronic arrangement of al and o atoms. Aluminum oxide, also known as alumina, is an inorganic compound composed of aluminum and oxygen. It is represented by the chemical. It is amphoteric in nature, and is used in various chemical, industrial and commercial applications. Aluminum oxide has a chemical formula al2o3. Aluminium oxide reacts with hot dilute hydrochloric. Aluminum Oxide Ionic Formula.
From www.slideserve.com
PPT Compounds PowerPoint Presentation, free download ID4539778 Aluminum Oxide Ionic Formula Aluminium oxide is one of the common ingredients in sunscreen and is also. A video explanation of how to write the chemical formula for aluminum oxide. Aluminum oxide, also known as alumina, is an inorganic compound composed of aluminum and oxygen. Aluminum oxide has a chemical formula al2o3. Let us see how we got the answer; It is represented by. Aluminum Oxide Ionic Formula.
From slidetodoc.com
Ionic Bonding Elements are the simplest substances There Aluminum Oxide Ionic Formula Aluminium oxide reacts with hot dilute hydrochloric acid to give aluminium chloride solution. The correct answer is #al_2o_3#. Aluminum oxide, with the chemical formula \ (al_2o_3\), is an amphoteric oxide and is commonly referred to as alumina. It is considered an indirect additive. Look at the electronic arrangement of al and o atoms. Aluminum oxide has a chemical formula al2o3.. Aluminum Oxide Ionic Formula.
From www.slideserve.com
PPT What are bonds? PowerPoint Presentation, free download ID5861644 Aluminum Oxide Ionic Formula Aluminium oxide reacts with hot dilute hydrochloric acid to give aluminium chloride solution. Aluminum oxide, also known as alumina, is an inorganic compound composed of aluminum and oxygen. Aluminium oxide is one of the common ingredients in sunscreen and is also. It is amphoteric in nature, and is used in various chemical, industrial and commercial applications. Aluminum oxide, with the. Aluminum Oxide Ionic Formula.
From chemistry291.blogspot.com
What Is the Chemical Formula for Aluminum Oxide? Aluminum Oxide Ionic Formula Aluminium oxide reacts with hot dilute hydrochloric acid to give aluminium chloride solution. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. Aluminium oxide (al 2 o 3 ) uses. Aluminum oxide, with the chemical formula \ (al_2o_3\), is an amphoteric oxide and is commonly referred to as alumina. Look. Aluminum Oxide Ionic Formula.
From www.slideserve.com
PPT Ch. 15 and 6 Naming and Writing Formulas for Ionic Compounds Aluminum Oxide Ionic Formula A video explanation of how to write the chemical formula for aluminum oxide. It is amphoteric in nature, and is used in various chemical, industrial and commercial applications. Aluminium oxide reacts with hot dilute hydrochloric acid to give aluminium chloride solution. Look at the electronic arrangement of al and o atoms. Aluminium oxide contains oxide ions, and thus reacts with. Aluminum Oxide Ionic Formula.
From ar.inspiredpencil.com
Aluminum Oxide Lewis Structure Aluminum Oxide Ionic Formula A video explanation of how to write the chemical formula for aluminum oxide. Look at the electronic arrangement of al and o atoms. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. It is amphoteric in nature, and is used in various chemical, industrial and commercial applications. Aluminium oxide is. Aluminum Oxide Ionic Formula.
From robot.ekstrabladet.dk
Formula Oxido De Aluminio Aluminum Oxide Ionic Formula Aluminum oxide, with the chemical formula \ (al_2o_3\), is an amphoteric oxide and is commonly referred to as alumina. The correct answer is #al_2o_3#. Look at the electronic arrangement of al and o atoms. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. Aluminium oxide contains oxide ions,. Aluminum Oxide Ionic Formula.
From www.youtube.com
Writing the Formula for Aluminum Oxide YouTube Aluminum Oxide Ionic Formula Aluminium oxide reacts with hot dilute hydrochloric acid to give aluminium chloride solution. Aluminum oxide, also known as alumina, is an inorganic compound composed of aluminum and oxygen. It is amphoteric in nature, and is used in various chemical, industrial and commercial applications. Aluminium oxide (al 2 o 3 ) uses. A video explanation of how to write the chemical. Aluminum Oxide Ionic Formula.
From manuallistcantabank.z21.web.core.windows.net
Al2o3 Lewis Diagram Aluminum Oxide Ionic Formula It is considered an indirect additive. A video explanation of how to write the chemical formula for aluminum oxide. Aluminium oxide (al 2 o 3 ) uses. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. Aluminium oxide reacts with hot dilute hydrochloric acid to give aluminium chloride. Aluminum Oxide Ionic Formula.
From eurassignmentcot.web.fc2.com
How to write aluminum oxide as a formula Aluminum Oxide Ionic Formula It is amphoteric in nature, and is used in various chemical, industrial and commercial applications. Let us see how we got the answer; Aluminium oxide (al 2 o 3 ) uses. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. The correct answer is #al_2o_3#. Aluminum oxide, with the chemical. Aluminum Oxide Ionic Formula.
From www.youtube.com
How To Draw The Lewis Structures of Ionic Compounds YouTube Aluminum Oxide Ionic Formula It is considered an indirect additive. A video explanation of how to write the chemical formula for aluminum oxide. Aluminium oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. Let us see how we got the answer; It is represented by the chemical. Aluminum oxide has a chemical formula al2o3. It. Aluminum Oxide Ionic Formula.
From stonecoldhands.com
Ionic Compounds Aluminum Oxide Ionic Formula Aluminum oxide, also known as alumina, is an inorganic compound composed of aluminum and oxygen. The correct answer is #al_2o_3#. Aluminum oxide, with the chemical formula \ (al_2o_3\), is an amphoteric oxide and is commonly referred to as alumina. A video explanation of how to write the chemical formula for aluminum oxide. Aluminium oxide (al 2 o 3 ) uses.. Aluminum Oxide Ionic Formula.