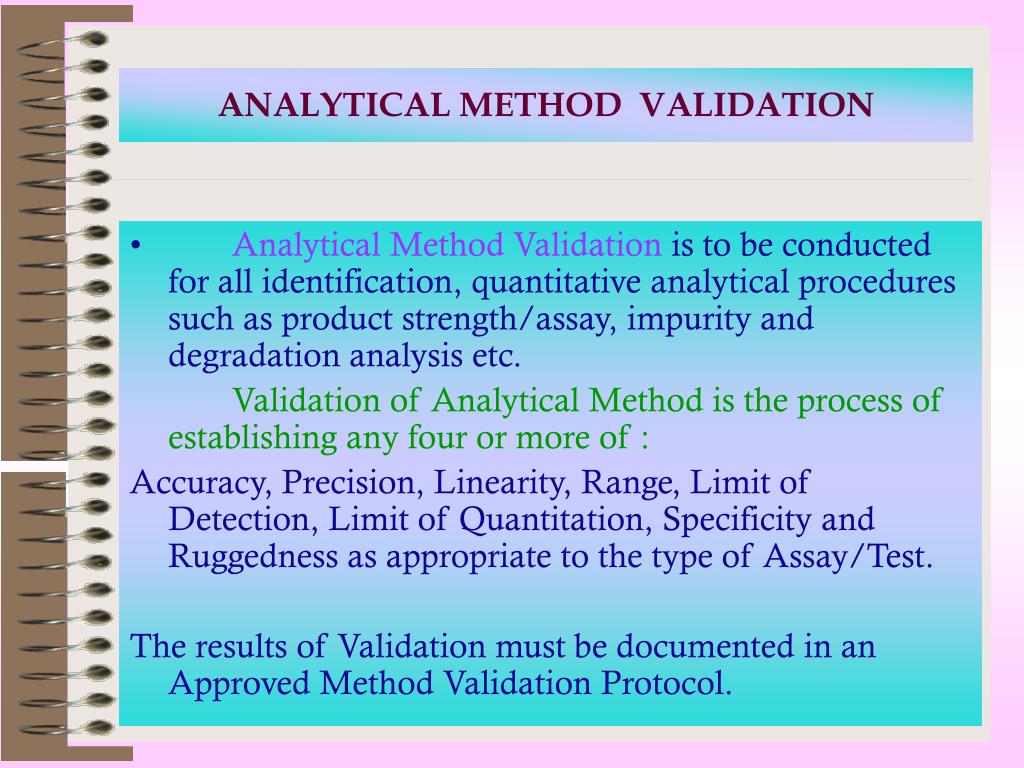
Analytical Verification Vs Validation . Although the measures to improve quality in the clinical laboratory have been enormous in the past years, not least of all due. Analytical method verification is usually required when a compendial method or a previously validated method is being performed with new or different products, equipment,. This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. This guideline presents a discussion of elements for consideration during the validation of analytical procedures included. This framework includes verification, analytical validation, and clinical validation (v3).
from www.slideserve.com
This framework includes verification, analytical validation, and clinical validation (v3). Although the measures to improve quality in the clinical laboratory have been enormous in the past years, not least of all due. This guideline presents a discussion of elements for consideration during the validation of analytical procedures included. This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. Analytical method verification is usually required when a compendial method or a previously validated method is being performed with new or different products, equipment,.
PPT VALIDATION METHODOLOGY PowerPoint Presentation, free download
Analytical Verification Vs Validation This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. This framework includes verification, analytical validation, and clinical validation (v3). This guideline presents a discussion of elements for consideration during the validation of analytical procedures included. Although the measures to improve quality in the clinical laboratory have been enormous in the past years, not least of all due. Analytical method verification is usually required when a compendial method or a previously validated method is being performed with new or different products, equipment,.
From elsmar.com
Verification vs. Validation Analytical Verification Vs Validation Analytical method verification is usually required when a compendial method or a previously validated method is being performed with new or different products, equipment,. This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. This guideline presents a discussion of elements for consideration during the validation of. Analytical Verification Vs Validation.
From www.youtube.com
SE 49 Verification VS Validation with Example YouTube Analytical Verification Vs Validation Analytical method verification is usually required when a compendial method or a previously validated method is being performed with new or different products, equipment,. This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. Although the measures to improve quality in the clinical laboratory have been enormous. Analytical Verification Vs Validation.
From www.youtube.com
Verification vs Validation testing Explained with example YouTube Analytical Verification Vs Validation This framework includes verification, analytical validation, and clinical validation (v3). This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. This guideline presents a discussion of elements for consideration during the validation of analytical procedures included. Although the measures to improve quality in the clinical laboratory have. Analytical Verification Vs Validation.
From qacraft.com
Validation Vs Verification Infographics Analytical Verification Vs Validation Analytical method verification is usually required when a compendial method or a previously validated method is being performed with new or different products, equipment,. This guideline presents a discussion of elements for consideration during the validation of analytical procedures included. Although the measures to improve quality in the clinical laboratory have been enormous in the past years, not least of. Analytical Verification Vs Validation.
From www.eventura.us
Exploring Analytical Method Validation and Verification vs. Validation Analytical Verification Vs Validation Although the measures to improve quality in the clinical laboratory have been enormous in the past years, not least of all due. Analytical method verification is usually required when a compendial method or a previously validated method is being performed with new or different products, equipment,. This guideline presents a discussion of elements for consideration during the validation of analytical. Analytical Verification Vs Validation.
From tutorialshut.com
Difference between Validation and Verification Tutorials Hut Analytical Verification Vs Validation Although the measures to improve quality in the clinical laboratory have been enormous in the past years, not least of all due. This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. This framework includes verification, analytical validation, and clinical validation (v3). Analytical method verification is usually. Analytical Verification Vs Validation.
From www.lambdatest.com
Verification vs Validation Know The Differences in Testing Analytical Verification Vs Validation Although the measures to improve quality in the clinical laboratory have been enormous in the past years, not least of all due. Analytical method verification is usually required when a compendial method or a previously validated method is being performed with new or different products, equipment,. This guideline presents a discussion of elements for consideration during the validation of analytical. Analytical Verification Vs Validation.
From www.slideshare.net
Validation vs. verification Analytical Verification Vs Validation This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. This guideline presents a discussion of elements for consideration during the validation of analytical procedures included. Although the measures to improve quality in the clinical laboratory have been enormous in the past years, not least of all. Analytical Verification Vs Validation.
From www.slideshare.net
analytical method validation and validation of hplc Analytical Verification Vs Validation This guideline presents a discussion of elements for consideration during the validation of analytical procedures included. Although the measures to improve quality in the clinical laboratory have been enormous in the past years, not least of all due. Analytical method verification is usually required when a compendial method or a previously validated method is being performed with new or different. Analytical Verification Vs Validation.
From www.johner-institute.nz
Validation vs Verification Johner Institute New Zealand Analytical Verification Vs Validation Analytical method verification is usually required when a compendial method or a previously validated method is being performed with new or different products, equipment,. This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. Although the measures to improve quality in the clinical laboratory have been enormous. Analytical Verification Vs Validation.
From www.slideserve.com
PPT VALIDATION METHODOLOGY PowerPoint Presentation, free download Analytical Verification Vs Validation This guideline presents a discussion of elements for consideration during the validation of analytical procedures included. This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. This framework includes verification, analytical validation, and clinical validation (v3). Analytical method verification is usually required when a compendial method or. Analytical Verification Vs Validation.
From www.researchgate.net
(PDF) Verification, Analytical Validation, and Clinical Validation (V3 Analytical Verification Vs Validation Although the measures to improve quality in the clinical laboratory have been enormous in the past years, not least of all due. Analytical method verification is usually required when a compendial method or a previously validated method is being performed with new or different products, equipment,. This review summarizes the current literature on the topic, focusing on the requirements for. Analytical Verification Vs Validation.
From www.researchgate.net
Logical flow diagram of verification and validation of analytical Analytical Verification Vs Validation This guideline presents a discussion of elements for consideration during the validation of analytical procedures included. This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. Analytical method verification is usually required when a compendial method or a previously validated method is being performed with new or. Analytical Verification Vs Validation.
From techblogs.42gears.com
Verification and Validation in Software Testing Tech Blogs Analytical Verification Vs Validation This guideline presents a discussion of elements for consideration during the validation of analytical procedures included. Although the measures to improve quality in the clinical laboratory have been enormous in the past years, not least of all due. This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be,. Analytical Verification Vs Validation.
From thecontentauthority.com
Verification vs Validation When To Use Each One In Writing Analytical Verification Vs Validation This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. This guideline presents a discussion of elements for consideration during the validation of analytical procedures included. Analytical method verification is usually required when a compendial method or a previously validated method is being performed with new or. Analytical Verification Vs Validation.
From testsigma.com
Verification vs Validation Testing Key Differences Analytical Verification Vs Validation This framework includes verification, analytical validation, and clinical validation (v3). This guideline presents a discussion of elements for consideration during the validation of analytical procedures included. Analytical method verification is usually required when a compendial method or a previously validated method is being performed with new or different products, equipment,. Although the measures to improve quality in the clinical laboratory. Analytical Verification Vs Validation.
From npjdigitalmedcommunity.nature.com
Why V3 (verification, analytical validation, and clinical validation Analytical Verification Vs Validation Although the measures to improve quality in the clinical laboratory have been enormous in the past years, not least of all due. This framework includes verification, analytical validation, and clinical validation (v3). This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. This guideline presents a discussion. Analytical Verification Vs Validation.
From www.technolush.com
Verification & Validation Model TechnoLush Analytical Verification Vs Validation This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. This guideline presents a discussion of elements for consideration during the validation of analytical procedures included. Analytical method verification is usually required when a compendial method or a previously validated method is being performed with new or. Analytical Verification Vs Validation.
From www.slideserve.com
PPT A StepbyStep Guide for Method Validation PowerPoint Analytical Verification Vs Validation This framework includes verification, analytical validation, and clinical validation (v3). Although the measures to improve quality in the clinical laboratory have been enormous in the past years, not least of all due. This guideline presents a discussion of elements for consideration during the validation of analytical procedures included. This review summarizes the current literature on the topic, focusing on the. Analytical Verification Vs Validation.
From www.youtube.com
Verification vs. Validation Precision vs. Accuracy YouTube Analytical Verification Vs Validation This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. Although the measures to improve quality in the clinical laboratory have been enormous in the past years, not least of all due. Analytical method verification is usually required when a compendial method or a previously validated method. Analytical Verification Vs Validation.
From www.researchgate.net
Verification Versus Validation Download Scientific Diagram Analytical Verification Vs Validation Analytical method verification is usually required when a compendial method or a previously validated method is being performed with new or different products, equipment,. This guideline presents a discussion of elements for consideration during the validation of analytical procedures included. This framework includes verification, analytical validation, and clinical validation (v3). This review summarizes the current literature on the topic, focusing. Analytical Verification Vs Validation.
From slidetodoc.com
Analysis 1 Verification vs Validation Verification Validation Are Analytical Verification Vs Validation This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. This framework includes verification, analytical validation, and clinical validation (v3). This guideline presents a discussion of elements for consideration during the validation of analytical procedures included. Although the measures to improve quality in the clinical laboratory have. Analytical Verification Vs Validation.
From www.researchgate.net
V3 in practice The verification, analytical validation, and clinical Analytical Verification Vs Validation This framework includes verification, analytical validation, and clinical validation (v3). Analytical method verification is usually required when a compendial method or a previously validated method is being performed with new or different products, equipment,. This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. Although the measures. Analytical Verification Vs Validation.
From www.unifiedinfotech.net
What are Software Verification and Validation Testing? Analytical Verification Vs Validation Although the measures to improve quality in the clinical laboratory have been enormous in the past years, not least of all due. Analytical method verification is usually required when a compendial method or a previously validated method is being performed with new or different products, equipment,. This guideline presents a discussion of elements for consideration during the validation of analytical. Analytical Verification Vs Validation.
From www.linkedin.com
What Is The Difference Between Verification And Validation Of Analytical Verification Vs Validation This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. This guideline presents a discussion of elements for consideration during the validation of analytical procedures included. This framework includes verification, analytical validation, and clinical validation (v3). Although the measures to improve quality in the clinical laboratory have. Analytical Verification Vs Validation.
From www.softwaretestingmaterial.com
What is Verification And Validation In Software Testing Analytical Verification Vs Validation This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. This guideline presents a discussion of elements for consideration during the validation of analytical procedures included. Analytical method verification is usually required when a compendial method or a previously validated method is being performed with new or. Analytical Verification Vs Validation.
From www.semanticscholar.org
Validation and verification of measurement methods in clinical Analytical Verification Vs Validation This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. This framework includes verification, analytical validation, and clinical validation (v3). Although the measures to improve quality in the clinical laboratory have been enormous in the past years, not least of all due. Analytical method verification is usually. Analytical Verification Vs Validation.
From www.lambdatest.com
Verification vs Validation Know The Differences in Testing Analytical Verification Vs Validation Although the measures to improve quality in the clinical laboratory have been enormous in the past years, not least of all due. This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. This guideline presents a discussion of elements for consideration during the validation of analytical procedures. Analytical Verification Vs Validation.
From www.sentrakalibrasiindustri.com
Kapan Melakukan Verifikasi dan Validasi Metode Analisis? Analytical Verification Vs Validation Although the measures to improve quality in the clinical laboratory have been enormous in the past years, not least of all due. This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. This guideline presents a discussion of elements for consideration during the validation of analytical procedures. Analytical Verification Vs Validation.
From www.slideserve.com
PPT Validation of Analytical Methods PowerPoint Presentation, free Analytical Verification Vs Validation Analytical method verification is usually required when a compendial method or a previously validated method is being performed with new or different products, equipment,. This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. This framework includes verification, analytical validation, and clinical validation (v3). This guideline presents. Analytical Verification Vs Validation.
From understandingdata.com
What is Data Validation and When Do You Do It? Just Understanding Data Analytical Verification Vs Validation Although the measures to improve quality in the clinical laboratory have been enormous in the past years, not least of all due. This framework includes verification, analytical validation, and clinical validation (v3). Analytical method verification is usually required when a compendial method or a previously validated method is being performed with new or different products, equipment,. This guideline presents a. Analytical Verification Vs Validation.
From en.ppt-online.org
Method Validation and Verification Protocols for Test Methods online Analytical Verification Vs Validation This framework includes verification, analytical validation, and clinical validation (v3). This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. Although the measures to improve quality in the clinical laboratory have been enormous in the past years, not least of all due. Analytical method verification is usually. Analytical Verification Vs Validation.
From infyom.com
Verification and Validation What’s the difference? Analytical Verification Vs Validation Analytical method verification is usually required when a compendial method or a previously validated method is being performed with new or different products, equipment,. This framework includes verification, analytical validation, and clinical validation (v3). This guideline presents a discussion of elements for consideration during the validation of analytical procedures included. This review summarizes the current literature on the topic, focusing. Analytical Verification Vs Validation.
From www.slideserve.com
PPT Analytical Methods What, When and How to Validate PowerPoint Analytical Verification Vs Validation This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. This framework includes verification, analytical validation, and clinical validation (v3). Analytical method verification is usually required when a compendial method or a previously validated method is being performed with new or different products, equipment,. This guideline presents. Analytical Verification Vs Validation.
From www.youtube.com
Verification vs Validation in Software Testing 4 Manual Testing Analytical Verification Vs Validation This framework includes verification, analytical validation, and clinical validation (v3). Analytical method verification is usually required when a compendial method or a previously validated method is being performed with new or different products, equipment,. This review summarizes the current literature on the topic, focusing on the requirements for method validations, or as the case may be, verifications. Although the measures. Analytical Verification Vs Validation.