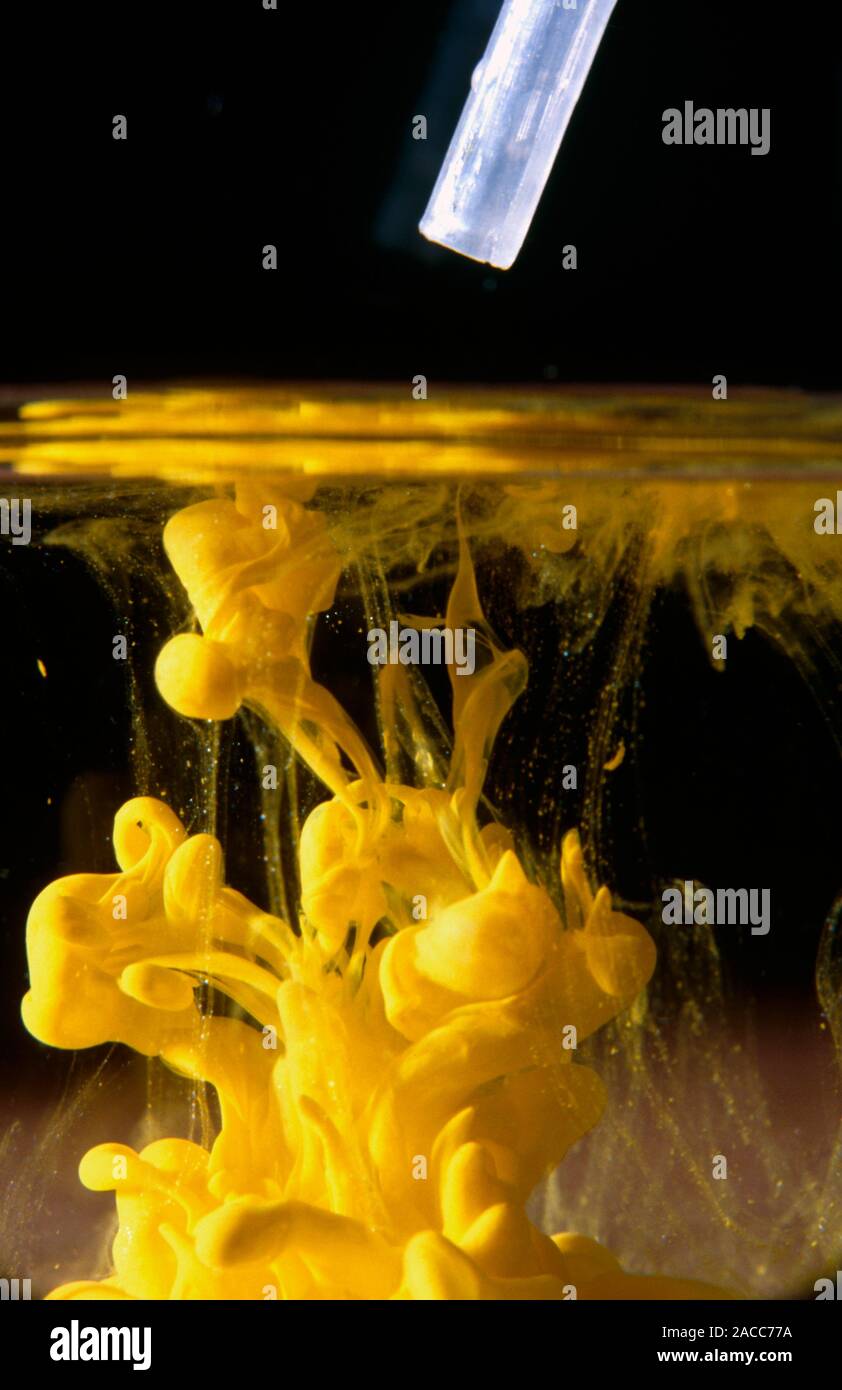
Lead Iodide Expression . It is formed by mixing by mixing lead (ii) nitrate and potassium iodide. Find the formula, molecular weight, cas registry number, and enthalpy and entropy of formation of lead iodide (ipb) in the gas phase. Learn about lead (ii) iodide, an ionic compound with the formula pbi2 and a bright yellow color. Lead iodide is a iodide of lead that varies in color from yellow to red, depending of the temperature. Write the dissolution equation and the solubility product expression for each of the following slightly soluble ionic compounds: Lead iodide is a chemical compound with the formula i 4 pb. Find out how it's used as an indicator for. The optical properties of lead iodide have been measured and the fundamental absorption spectrum is interpreted in terms of.
from www.alamy.com
Find the formula, molecular weight, cas registry number, and enthalpy and entropy of formation of lead iodide (ipb) in the gas phase. Find out how it's used as an indicator for. Learn about lead (ii) iodide, an ionic compound with the formula pbi2 and a bright yellow color. The optical properties of lead iodide have been measured and the fundamental absorption spectrum is interpreted in terms of. It is formed by mixing by mixing lead (ii) nitrate and potassium iodide. Write the dissolution equation and the solubility product expression for each of the following slightly soluble ionic compounds: Lead iodide is a chemical compound with the formula i 4 pb. Lead iodide is a iodide of lead that varies in color from yellow to red, depending of the temperature.
Precipitation of lead iodide. A yellow precipitate of lead iodide
Lead Iodide Expression The optical properties of lead iodide have been measured and the fundamental absorption spectrum is interpreted in terms of. Find the formula, molecular weight, cas registry number, and enthalpy and entropy of formation of lead iodide (ipb) in the gas phase. Lead iodide is a chemical compound with the formula i 4 pb. Find out how it's used as an indicator for. Lead iodide is a iodide of lead that varies in color from yellow to red, depending of the temperature. The optical properties of lead iodide have been measured and the fundamental absorption spectrum is interpreted in terms of. It is formed by mixing by mixing lead (ii) nitrate and potassium iodide. Write the dissolution equation and the solubility product expression for each of the following slightly soluble ionic compounds: Learn about lead (ii) iodide, an ionic compound with the formula pbi2 and a bright yellow color.
From alannahminhardy.blogspot.com
Particle Diagram of Lead Nitrate and Potassium Iodide AlannahminHardy Lead Iodide Expression Lead iodide is a iodide of lead that varies in color from yellow to red, depending of the temperature. Learn about lead (ii) iodide, an ionic compound with the formula pbi2 and a bright yellow color. The optical properties of lead iodide have been measured and the fundamental absorption spectrum is interpreted in terms of. Lead iodide is a chemical. Lead Iodide Expression.
From www.numerade.com
SOLVED a)Lead iodide is created by reacting lead nitrate with Lead Iodide Expression Write the dissolution equation and the solubility product expression for each of the following slightly soluble ionic compounds: Learn about lead (ii) iodide, an ionic compound with the formula pbi2 and a bright yellow color. The optical properties of lead iodide have been measured and the fundamental absorption spectrum is interpreted in terms of. Find the formula, molecular weight, cas. Lead Iodide Expression.
From www.youtube.com
Lead Iodide Precipitating YouTube Lead Iodide Expression It is formed by mixing by mixing lead (ii) nitrate and potassium iodide. Lead iodide is a chemical compound with the formula i 4 pb. Find out how it's used as an indicator for. The optical properties of lead iodide have been measured and the fundamental absorption spectrum is interpreted in terms of. Lead iodide is a iodide of lead. Lead Iodide Expression.
From www.pw.live
Lead Iodide Formula, Structure And Molar Mass Lead Iodide Expression Lead iodide is a iodide of lead that varies in color from yellow to red, depending of the temperature. Find out how it's used as an indicator for. Lead iodide is a chemical compound with the formula i 4 pb. Write the dissolution equation and the solubility product expression for each of the following slightly soluble ionic compounds: The optical. Lead Iodide Expression.
From www.researchgate.net
SEM images of the 1D lead iodide hybrid Download Scientific Diagram Lead Iodide Expression Lead iodide is a chemical compound with the formula i 4 pb. Lead iodide is a iodide of lead that varies in color from yellow to red, depending of the temperature. The optical properties of lead iodide have been measured and the fundamental absorption spectrum is interpreted in terms of. Learn about lead (ii) iodide, an ionic compound with the. Lead Iodide Expression.
From fineartamerica.com
Lead Iodide Reaction Photograph by Dorling Kindersley Fine Art America Lead Iodide Expression Learn about lead (ii) iodide, an ionic compound with the formula pbi2 and a bright yellow color. Lead iodide is a chemical compound with the formula i 4 pb. Write the dissolution equation and the solubility product expression for each of the following slightly soluble ionic compounds: It is formed by mixing by mixing lead (ii) nitrate and potassium iodide.. Lead Iodide Expression.
From www.chemborun.com
Lead iodide PbI2 Perovskitebased Photodiodes (PPD) Materials Lead Iodide Expression Lead iodide is a iodide of lead that varies in color from yellow to red, depending of the temperature. Learn about lead (ii) iodide, an ionic compound with the formula pbi2 and a bright yellow color. Find out how it's used as an indicator for. Find the formula, molecular weight, cas registry number, and enthalpy and entropy of formation of. Lead Iodide Expression.
From www.researchgate.net
The supercell model of lead iodide (a) containing 96 atoms or (b Lead Iodide Expression Lead iodide is a iodide of lead that varies in color from yellow to red, depending of the temperature. Write the dissolution equation and the solubility product expression for each of the following slightly soluble ionic compounds: Learn about lead (ii) iodide, an ionic compound with the formula pbi2 and a bright yellow color. Lead iodide is a chemical compound. Lead Iodide Expression.
From pixels.com
Formation Of Lead (ii) Iodide Photograph by Martyn F. Chillmaid/science Lead Iodide Expression Find the formula, molecular weight, cas registry number, and enthalpy and entropy of formation of lead iodide (ipb) in the gas phase. Write the dissolution equation and the solubility product expression for each of the following slightly soluble ionic compounds: Lead iodide is a iodide of lead that varies in color from yellow to red, depending of the temperature. Lead. Lead Iodide Expression.
From www.researchgate.net
Image of methyl ammonium lead iodide perovskite/PANI [266] Download Lead Iodide Expression It is formed by mixing by mixing lead (ii) nitrate and potassium iodide. Write the dissolution equation and the solubility product expression for each of the following slightly soluble ionic compounds: Lead iodide is a iodide of lead that varies in color from yellow to red, depending of the temperature. Find the formula, molecular weight, cas registry number, and enthalpy. Lead Iodide Expression.
From webapi.bu.edu
Lead iodide formula. Lead Iodide Structure, Preparations and Lead Iodide Expression Lead iodide is a iodide of lead that varies in color from yellow to red, depending of the temperature. The optical properties of lead iodide have been measured and the fundamental absorption spectrum is interpreted in terms of. It is formed by mixing by mixing lead (ii) nitrate and potassium iodide. Learn about lead (ii) iodide, an ionic compound with. Lead Iodide Expression.
From webapi.bu.edu
Lead iodide formula. Lead Iodide Structure, Preparations and Lead Iodide Expression Learn about lead (ii) iodide, an ionic compound with the formula pbi2 and a bright yellow color. It is formed by mixing by mixing lead (ii) nitrate and potassium iodide. Find out how it's used as an indicator for. The optical properties of lead iodide have been measured and the fundamental absorption spectrum is interpreted in terms of. Write the. Lead Iodide Expression.
From www.numerade.com
SOLVED 2) For the insoluble salt lead(II) iodide, the solubility Lead Iodide Expression Learn about lead (ii) iodide, an ionic compound with the formula pbi2 and a bright yellow color. Lead iodide is a chemical compound with the formula i 4 pb. It is formed by mixing by mixing lead (ii) nitrate and potassium iodide. Lead iodide is a iodide of lead that varies in color from yellow to red, depending of the. Lead Iodide Expression.
From www.scribd.com
Ksp of Lead Iodide Solubility Physical Sciences Lead Iodide Expression Write the dissolution equation and the solubility product expression for each of the following slightly soluble ionic compounds: Lead iodide is a iodide of lead that varies in color from yellow to red, depending of the temperature. Find out how it's used as an indicator for. Lead iodide is a chemical compound with the formula i 4 pb. The optical. Lead Iodide Expression.
From www.shutterstock.com
Leadii Iodide Lead Iodide Formula Pbi2 Stock Illustration 729111571 Lead Iodide Expression Lead iodide is a iodide of lead that varies in color from yellow to red, depending of the temperature. Find out how it's used as an indicator for. The optical properties of lead iodide have been measured and the fundamental absorption spectrum is interpreted in terms of. Write the dissolution equation and the solubility product expression for each of the. Lead Iodide Expression.
From pixels.com
Lead Iodide Precipitate Photograph by Andrew Lambert Photography Lead Iodide Expression Lead iodide is a chemical compound with the formula i 4 pb. Find out how it's used as an indicator for. The optical properties of lead iodide have been measured and the fundamental absorption spectrum is interpreted in terms of. Learn about lead (ii) iodide, an ionic compound with the formula pbi2 and a bright yellow color. Write the dissolution. Lead Iodide Expression.
From www.numerade.com
SOLVED 13.A student makes saturated solution of lead (II) iodide, Pblz Lead Iodide Expression Lead iodide is a iodide of lead that varies in color from yellow to red, depending of the temperature. Write the dissolution equation and the solubility product expression for each of the following slightly soluble ionic compounds: Learn about lead (ii) iodide, an ionic compound with the formula pbi2 and a bright yellow color. Find the formula, molecular weight, cas. Lead Iodide Expression.
From www.flickr.com
Lead Iodide Pittsburgh Carnegie Science and Trib Total Med… Flickr Lead Iodide Expression Lead iodide is a chemical compound with the formula i 4 pb. The optical properties of lead iodide have been measured and the fundamental absorption spectrum is interpreted in terms of. Lead iodide is a iodide of lead that varies in color from yellow to red, depending of the temperature. Write the dissolution equation and the solubility product expression for. Lead Iodide Expression.
From www.numerade.com
⏩SOLVEDWrite the ionproduct expressions for (a) lead(II) iodide Lead Iodide Expression Lead iodide is a chemical compound with the formula i 4 pb. Lead iodide is a iodide of lead that varies in color from yellow to red, depending of the temperature. It is formed by mixing by mixing lead (ii) nitrate and potassium iodide. Find out how it's used as an indicator for. Find the formula, molecular weight, cas registry. Lead Iodide Expression.
From www.researchgate.net
a lead iodide is first spuncast onto the substrates to form dense and Lead Iodide Expression Learn about lead (ii) iodide, an ionic compound with the formula pbi2 and a bright yellow color. It is formed by mixing by mixing lead (ii) nitrate and potassium iodide. Lead iodide is a iodide of lead that varies in color from yellow to red, depending of the temperature. The optical properties of lead iodide have been measured and the. Lead Iodide Expression.
From www.researchgate.net
2) a) Lead iodide purification setup and stages, and b) produced Lead Iodide Expression Lead iodide is a iodide of lead that varies in color from yellow to red, depending of the temperature. Lead iodide is a chemical compound with the formula i 4 pb. It is formed by mixing by mixing lead (ii) nitrate and potassium iodide. Find the formula, molecular weight, cas registry number, and enthalpy and entropy of formation of lead. Lead Iodide Expression.
From www.alamy.com
Precipitation of lead iodide. A yellow precipitate of lead iodide Lead Iodide Expression Find out how it's used as an indicator for. Write the dissolution equation and the solubility product expression for each of the following slightly soluble ionic compounds: Lead iodide is a chemical compound with the formula i 4 pb. The optical properties of lead iodide have been measured and the fundamental absorption spectrum is interpreted in terms of. It is. Lead Iodide Expression.
From www.compoundchem.com
Compound Interest Chemical Reactions Lead Iodide & ‘Golden Rain’ Lead Iodide Expression It is formed by mixing by mixing lead (ii) nitrate and potassium iodide. Lead iodide is a chemical compound with the formula i 4 pb. The optical properties of lead iodide have been measured and the fundamental absorption spectrum is interpreted in terms of. Find the formula, molecular weight, cas registry number, and enthalpy and entropy of formation of lead. Lead Iodide Expression.
From www.numerade.com
SOLVEDWrite the ionproduct expressions for (a) lead(II) iodide; (b Lead Iodide Expression Learn about lead (ii) iodide, an ionic compound with the formula pbi2 and a bright yellow color. It is formed by mixing by mixing lead (ii) nitrate and potassium iodide. Write the dissolution equation and the solubility product expression for each of the following slightly soluble ionic compounds: Find the formula, molecular weight, cas registry number, and enthalpy and entropy. Lead Iodide Expression.
From woelen.homescience.net
Science made alive Lead Iodide Expression Lead iodide is a iodide of lead that varies in color from yellow to red, depending of the temperature. Lead iodide is a chemical compound with the formula i 4 pb. Find the formula, molecular weight, cas registry number, and enthalpy and entropy of formation of lead iodide (ipb) in the gas phase. Learn about lead (ii) iodide, an ionic. Lead Iodide Expression.
From www.researchgate.net
SEM images of lead iodide films formed from coatings of formulations Lead Iodide Expression It is formed by mixing by mixing lead (ii) nitrate and potassium iodide. Learn about lead (ii) iodide, an ionic compound with the formula pbi2 and a bright yellow color. Write the dissolution equation and the solubility product expression for each of the following slightly soluble ionic compounds: Find the formula, molecular weight, cas registry number, and enthalpy and entropy. Lead Iodide Expression.
From achs-prod.acs.org
Phase Behavior and Polymorphism of Formamidinium Lead Iodide Lead Iodide Expression Find out how it's used as an indicator for. Lead iodide is a chemical compound with the formula i 4 pb. Learn about lead (ii) iodide, an ionic compound with the formula pbi2 and a bright yellow color. The optical properties of lead iodide have been measured and the fundamental absorption spectrum is interpreted in terms of. It is formed. Lead Iodide Expression.
From www.compoundchem.com
Chemical Reactions Lead Iodide & 'Golden Rain' Compound Interest Lead Iodide Expression Find the formula, molecular weight, cas registry number, and enthalpy and entropy of formation of lead iodide (ipb) in the gas phase. It is formed by mixing by mixing lead (ii) nitrate and potassium iodide. Write the dissolution equation and the solubility product expression for each of the following slightly soluble ionic compounds: Lead iodide is a chemical compound with. Lead Iodide Expression.
From stock.adobe.com
Lead Iodide Properties and Chemical Compound Structure Vector Design Lead Iodide Expression Lead iodide is a iodide of lead that varies in color from yellow to red, depending of the temperature. Find out how it's used as an indicator for. Learn about lead (ii) iodide, an ionic compound with the formula pbi2 and a bright yellow color. Find the formula, molecular weight, cas registry number, and enthalpy and entropy of formation of. Lead Iodide Expression.
From depositphotos.com
Lead Iodide Properties Chemical Compound Structure Stock Vector Image Lead Iodide Expression Lead iodide is a iodide of lead that varies in color from yellow to red, depending of the temperature. Find the formula, molecular weight, cas registry number, and enthalpy and entropy of formation of lead iodide (ipb) in the gas phase. The optical properties of lead iodide have been measured and the fundamental absorption spectrum is interpreted in terms of.. Lead Iodide Expression.
From www.youtube.com
How to write the formula for lead (IV) iodide YouTube Lead Iodide Expression The optical properties of lead iodide have been measured and the fundamental absorption spectrum is interpreted in terms of. Lead iodide is a chemical compound with the formula i 4 pb. Find out how it's used as an indicator for. Learn about lead (ii) iodide, an ionic compound with the formula pbi2 and a bright yellow color. Write the dissolution. Lead Iodide Expression.
From fphoto.photoshelter.com
science chemistry precipitation reaction lead iodide Fundamental Lead Iodide Expression Find out how it's used as an indicator for. The optical properties of lead iodide have been measured and the fundamental absorption spectrum is interpreted in terms of. Lead iodide is a iodide of lead that varies in color from yellow to red, depending of the temperature. Find the formula, molecular weight, cas registry number, and enthalpy and entropy of. Lead Iodide Expression.
From www.semanticscholar.org
Figure 2 from From Lead Iodide to a Radical Form LeadIodide Lead Iodide Expression Learn about lead (ii) iodide, an ionic compound with the formula pbi2 and a bright yellow color. Lead iodide is a iodide of lead that varies in color from yellow to red, depending of the temperature. Lead iodide is a chemical compound with the formula i 4 pb. Write the dissolution equation and the solubility product expression for each of. Lead Iodide Expression.
From testbook.com
Lead Iodide Formula Structure, Preparation Properties, Uses Lead Iodide Expression It is formed by mixing by mixing lead (ii) nitrate and potassium iodide. Lead iodide is a iodide of lead that varies in color from yellow to red, depending of the temperature. Find the formula, molecular weight, cas registry number, and enthalpy and entropy of formation of lead iodide (ipb) in the gas phase. Write the dissolution equation and the. Lead Iodide Expression.
From fphoto.photoshelter.com
science chemistry precipitation reaction lead iodide Fundamental Lead Iodide Expression The optical properties of lead iodide have been measured and the fundamental absorption spectrum is interpreted in terms of. Lead iodide is a chemical compound with the formula i 4 pb. Learn about lead (ii) iodide, an ionic compound with the formula pbi2 and a bright yellow color. It is formed by mixing by mixing lead (ii) nitrate and potassium. Lead Iodide Expression.