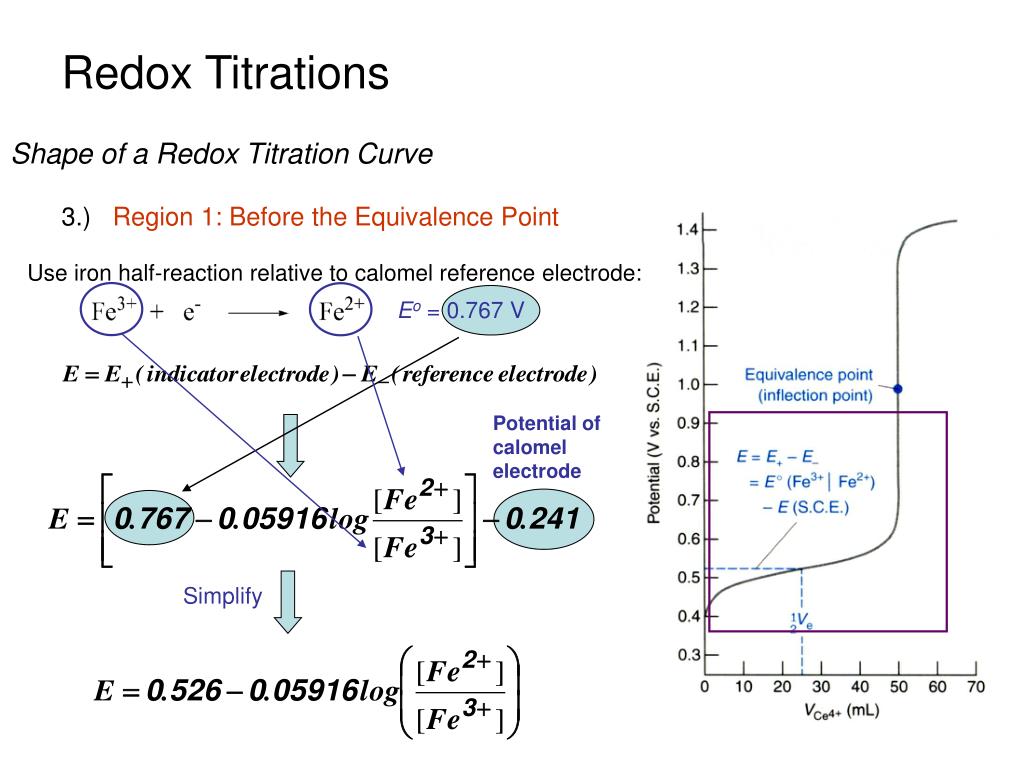
Redox Titration Introduction . This method relies on the. 4+ 2++ fe 3+ →. Redox titration determines the concentration of an unknown solution (analyte) that contains an oxidizing or reducing agent. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. To evaluate a redox titration we need to know the shape of its titration curve.
from www.slideserve.com
To evaluate a redox titration we need to know the shape of its titration curve. Redox titration determines the concentration of an unknown solution (analyte) that contains an oxidizing or reducing agent. 4+ 2++ fe 3+ →. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. This method relies on the.
PPT Redox Titrations PowerPoint Presentation, free download ID1154145
Redox Titration Introduction To evaluate a redox titration we need to know the shape of its titration curve. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. This method relies on the. Redox titration determines the concentration of an unknown solution (analyte) that contains an oxidizing or reducing agent. To evaluate a redox titration we need to know the shape of its titration curve. 4+ 2++ fe 3+ →.
From www.studypool.com
SOLUTION Redox titration and application in medicine and Redox Titration Introduction 4+ 2++ fe 3+ →. To evaluate a redox titration we need to know the shape of its titration curve. This method relies on the. Redox titration determines the concentration of an unknown solution (analyte) that contains an oxidizing or reducing agent. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox. Redox Titration Introduction.
From www.alamy.com
Scientist performing titration in a chemistry laboratory with Redox Redox Titration Introduction To evaluate a redox titration we need to know the shape of its titration curve. 4+ 2++ fe 3+ →. Redox titration determines the concentration of an unknown solution (analyte) that contains an oxidizing or reducing agent. This method relies on the. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox. Redox Titration Introduction.
From www.slideserve.com
PPT Redox Titrations PowerPoint Presentation, free download ID1154145 Redox Titration Introduction Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. Redox titration determines the concentration of an unknown solution (analyte) that contains an oxidizing or reducing agent. 4+ 2++ fe 3+ →. This method relies on the. To evaluate a redox titration we need to know the shape of. Redox Titration Introduction.
From www.youtube.com
Introduction to Redox Titration Titration Chemistry Iodimetry Redox Titration Introduction This method relies on the. To evaluate a redox titration we need to know the shape of its titration curve. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. Redox titration determines the concentration of an unknown solution (analyte) that contains an oxidizing or reducing agent. 4+ 2++. Redox Titration Introduction.
From www.vrogue.co
Redox Titrations Introduction 1 Redox Titration vrogue.co Redox Titration Introduction Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. Redox titration determines the concentration of an unknown solution (analyte) that contains an oxidizing or reducing agent. 4+ 2++ fe 3+ →. To evaluate a redox titration we need to know the shape of its titration curve. This method. Redox Titration Introduction.
From studylib.net
Determination of Iron by Redox Titration Redox Titration Introduction 4+ 2++ fe 3+ →. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. This method relies on the. Redox titration determines the concentration of an unknown solution (analyte) that contains an oxidizing or reducing agent. To evaluate a redox titration we need to know the shape of. Redox Titration Introduction.
From www.slideserve.com
PPT Redox Titrations PowerPoint Presentation, free download ID1154145 Redox Titration Introduction To evaluate a redox titration we need to know the shape of its titration curve. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. This method relies on the. Redox titration determines the concentration of an unknown solution (analyte) that contains an oxidizing or reducing agent. 4+ 2++. Redox Titration Introduction.
From www.slideserve.com
PPT Redox Titrations PowerPoint Presentation, free download ID1154145 Redox Titration Introduction Redox titration determines the concentration of an unknown solution (analyte) that contains an oxidizing or reducing agent. This method relies on the. To evaluate a redox titration we need to know the shape of its titration curve. 4+ 2++ fe 3+ →. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox. Redox Titration Introduction.
From www.youtube.com
Redox Titration Introduction YouTube Redox Titration Introduction Redox titration determines the concentration of an unknown solution (analyte) that contains an oxidizing or reducing agent. This method relies on the. 4+ 2++ fe 3+ →. To evaluate a redox titration we need to know the shape of its titration curve. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox. Redox Titration Introduction.
From www.studyread.com
Redox Titrations The Experimental steps and Applications Redox Titration Introduction 4+ 2++ fe 3+ →. This method relies on the. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. Redox titration determines the concentration of an unknown solution (analyte) that contains an oxidizing or reducing agent. To evaluate a redox titration we need to know the shape of. Redox Titration Introduction.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Redox Titration Introduction This method relies on the. Redox titration determines the concentration of an unknown solution (analyte) that contains an oxidizing or reducing agent. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. 4+ 2++ fe 3+ →. To evaluate a redox titration we need to know the shape of. Redox Titration Introduction.
From www.slideserve.com
PPT Redox Titration PowerPoint Presentation, free download ID583170 Redox Titration Introduction Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. Redox titration determines the concentration of an unknown solution (analyte) that contains an oxidizing or reducing agent. This method relies on the. To evaluate a redox titration we need to know the shape of its titration curve. 4+ 2++. Redox Titration Introduction.
From praxilabs.com
9 Most Effective Applications of Redox Titration PraxiLabs Redox Titration Introduction To evaluate a redox titration we need to know the shape of its titration curve. 4+ 2++ fe 3+ →. This method relies on the. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. Redox titration determines the concentration of an unknown solution (analyte) that contains an oxidizing. Redox Titration Introduction.
From www.studypool.com
SOLUTION Introduction redox titration Studypool Redox Titration Introduction Redox titration determines the concentration of an unknown solution (analyte) that contains an oxidizing or reducing agent. 4+ 2++ fe 3+ →. To evaluate a redox titration we need to know the shape of its titration curve. This method relies on the. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox. Redox Titration Introduction.
From www.youtube.com
Redox TitrationRedox reactionsredox titration experiment YouTube Redox Titration Introduction 4+ 2++ fe 3+ →. To evaluate a redox titration we need to know the shape of its titration curve. Redox titration determines the concentration of an unknown solution (analyte) that contains an oxidizing or reducing agent. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. This method. Redox Titration Introduction.
From pradeepsirpharmacy.blogspot.com
Redox Titrations Redox Titration Introduction To evaluate a redox titration we need to know the shape of its titration curve. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. Redox titration determines the concentration of an unknown solution (analyte) that contains an oxidizing or reducing agent. This method relies on the. 4+ 2++. Redox Titration Introduction.
From www.studypool.com
SOLUTION Introduction redox titration Studypool Redox Titration Introduction Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. 4+ 2++ fe 3+ →. Redox titration determines the concentration of an unknown solution (analyte) that contains an oxidizing or reducing agent. To evaluate a redox titration we need to know the shape of its titration curve. This method. Redox Titration Introduction.
From slidetodoc.com
Chapter 15 Redox Titrations 15 1 The Shape Redox Titration Introduction 4+ 2++ fe 3+ →. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. This method relies on the. Redox titration determines the concentration of an unknown solution (analyte) that contains an oxidizing or reducing agent. To evaluate a redox titration we need to know the shape of. Redox Titration Introduction.
From www.youtube.com
Redox titration calculations STEPbySTEP YouTube Redox Titration Introduction This method relies on the. Redox titration determines the concentration of an unknown solution (analyte) that contains an oxidizing or reducing agent. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. To evaluate a redox titration we need to know the shape of its titration curve. 4+ 2++. Redox Titration Introduction.
From www.youtube.com
Redox Titration Intro & Theory YouTube Redox Titration Introduction This method relies on the. Redox titration determines the concentration of an unknown solution (analyte) that contains an oxidizing or reducing agent. To evaluate a redox titration we need to know the shape of its titration curve. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. 4+ 2++. Redox Titration Introduction.
From www.youtube.com
Redox Titration Example Question 2 (Easy) ALevel Chemistry YouTube Redox Titration Introduction Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. 4+ 2++ fe 3+ →. This method relies on the. To evaluate a redox titration we need to know the shape of its titration curve. Redox titration determines the concentration of an unknown solution (analyte) that contains an oxidizing. Redox Titration Introduction.
From www.studypool.com
SOLUTION Introduction redox titration Studypool Redox Titration Introduction To evaluate a redox titration we need to know the shape of its titration curve. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. 4+ 2++ fe 3+ →. Redox titration determines the concentration of an unknown solution (analyte) that contains an oxidizing or reducing agent. This method. Redox Titration Introduction.
From www.slideserve.com
PPT Ch 16 Redox Titrations PowerPoint Presentation, free download Redox Titration Introduction This method relies on the. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. Redox titration determines the concentration of an unknown solution (analyte) that contains an oxidizing or reducing agent. 4+ 2++ fe 3+ →. To evaluate a redox titration we need to know the shape of. Redox Titration Introduction.
From www.slideserve.com
PPT Redox Titrations PowerPoint Presentation, free download ID3080571 Redox Titration Introduction 4+ 2++ fe 3+ →. Redox titration determines the concentration of an unknown solution (analyte) that contains an oxidizing or reducing agent. To evaluate a redox titration we need to know the shape of its titration curve. This method relies on the. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox. Redox Titration Introduction.
From www.chegg.com
Solved EXPERIMENT 13 REDOX TITRATION Introduction As we Redox Titration Introduction To evaluate a redox titration we need to know the shape of its titration curve. 4+ 2++ fe 3+ →. This method relies on the. Redox titration determines the concentration of an unknown solution (analyte) that contains an oxidizing or reducing agent. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox. Redox Titration Introduction.
From chem.libretexts.org
Redox Titration Chemistry LibreTexts Redox Titration Introduction To evaluate a redox titration we need to know the shape of its titration curve. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. This method relies on the. Redox titration determines the concentration of an unknown solution (analyte) that contains an oxidizing or reducing agent. 4+ 2++. Redox Titration Introduction.
From www.vrogue.co
Redox Titrations Introduction 1 Redox Titration vrogue.co Redox Titration Introduction 4+ 2++ fe 3+ →. Redox titration determines the concentration of an unknown solution (analyte) that contains an oxidizing or reducing agent. This method relies on the. To evaluate a redox titration we need to know the shape of its titration curve. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox. Redox Titration Introduction.
From www.youtube.com
REDOX REACTIONS AS THE BASIS FOR TITRATIONS YouTube Redox Titration Introduction Redox titration determines the concentration of an unknown solution (analyte) that contains an oxidizing or reducing agent. This method relies on the. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. To evaluate a redox titration we need to know the shape of its titration curve. 4+ 2++. Redox Titration Introduction.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Redox Titration Introduction This method relies on the. To evaluate a redox titration we need to know the shape of its titration curve. Redox titration determines the concentration of an unknown solution (analyte) that contains an oxidizing or reducing agent. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. 4+ 2++. Redox Titration Introduction.
From www.slideserve.com
PPT Redox Titrations PowerPoint Presentation, free download ID1154145 Redox Titration Introduction This method relies on the. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. Redox titration determines the concentration of an unknown solution (analyte) that contains an oxidizing or reducing agent. 4+ 2++ fe 3+ →. To evaluate a redox titration we need to know the shape of. Redox Titration Introduction.
From www.slideserve.com
PPT Redox Titrations PowerPoint Presentation, free download ID3080571 Redox Titration Introduction To evaluate a redox titration we need to know the shape of its titration curve. 4+ 2++ fe 3+ →. Redox titration determines the concentration of an unknown solution (analyte) that contains an oxidizing or reducing agent. This method relies on the. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox. Redox Titration Introduction.
From www.slideserve.com
PPT REDOX TITRATION PowerPoint Presentation ID431911 Redox Titration Introduction Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. Redox titration determines the concentration of an unknown solution (analyte) that contains an oxidizing or reducing agent. This method relies on the. 4+ 2++ fe 3+ →. To evaluate a redox titration we need to know the shape of. Redox Titration Introduction.
From www.slideserve.com
PPT Redox Stoichiometry PowerPoint Presentation, free download ID Redox Titration Introduction Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. To evaluate a redox titration we need to know the shape of its titration curve. 4+ 2++ fe 3+ →. This method relies on the. Redox titration determines the concentration of an unknown solution (analyte) that contains an oxidizing. Redox Titration Introduction.
From www.youtube.com
Redox Titration Introduction ALevel Chemistry YouTube Redox Titration Introduction Redox titration determines the concentration of an unknown solution (analyte) that contains an oxidizing or reducing agent. To evaluate a redox titration we need to know the shape of its titration curve. This method relies on the. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. 4+ 2++. Redox Titration Introduction.
From www.studocu.com
Redox Titration Intro Introduction Reduction / Oxidation chemistry Redox Titration Introduction To evaluate a redox titration we need to know the shape of its titration curve. Redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. 4+ 2++ fe 3+ →. This method relies on the. Redox titration determines the concentration of an unknown solution (analyte) that contains an oxidizing. Redox Titration Introduction.