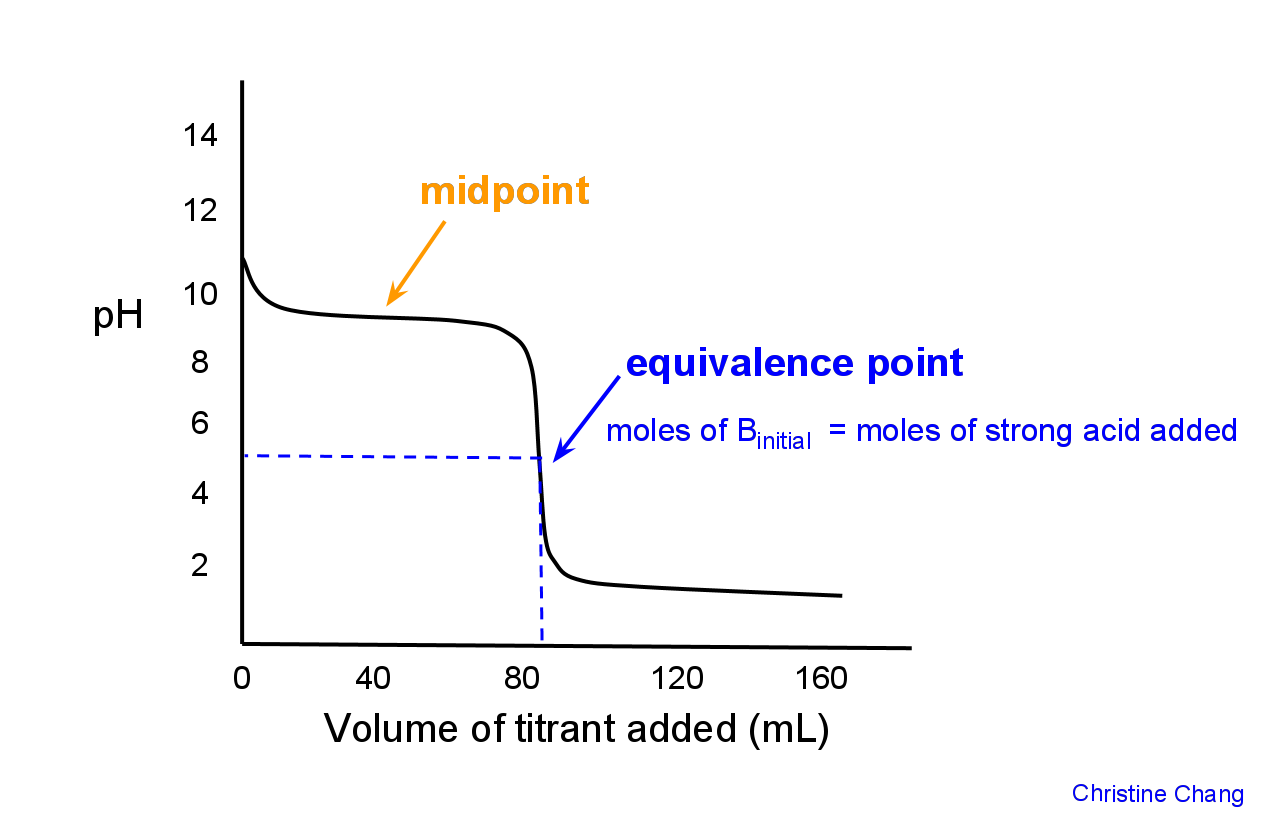
Titration Curve Shape Explained . a titration curve is a graphical representation of the ph of a solution during a titration. The way you normally carry out a titration involves adding the acid to the alkali. how do you explain the shape of a titration curve? a summary of the important curves. there are four shapes of titration curves that you need to be able to explain. a titration curve is a graphical representation of the ph of a solution during a titration. As the starting solution is. The figure below shows two different. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. Everything you need to know for a. In the examples shown below, alkali is added to an acid. And why is the equivalence point not always at ph7? the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what. On the left is a titration in which the base is added to the acid, and so the ph progresses from low to high.
from chem.libretexts.org
On the left is a titration in which the base is added to the acid, and so the ph progresses from low to high. a summary of the important curves. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. In the examples shown below, alkali is added to an acid. As the starting solution is. there are four shapes of titration curves that you need to be able to explain. a titration curve is a graphical representation of the ph of a solution during a titration. the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what. The figure below shows two different. And why is the equivalence point not always at ph7?
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts
Titration Curve Shape Explained And why is the equivalence point not always at ph7? In the examples shown below, alkali is added to an acid. Everything you need to know for a. a titration curve is a graphical representation of the ph of a solution during a titration. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. On the left is a titration in which the base is added to the acid, and so the ph progresses from low to high. As the starting solution is. there are four shapes of titration curves that you need to be able to explain. The way you normally carry out a titration involves adding the acid to the alkali. how do you explain the shape of a titration curve? And why is the equivalence point not always at ph7? a summary of the important curves. The figure below shows two different. a titration curve is a graphical representation of the ph of a solution during a titration. the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what.
From www.ck12.org
Titration Curve Overview ( Video ) Chemistry CK12 Foundation Titration Curve Shape Explained a titration curve is a graphical representation of the ph of a solution during a titration. a summary of the important curves. As the starting solution is. And why is the equivalence point not always at ph7? a titration curve is a graphical representation of the ph of a solution during a titration. how do you. Titration Curve Shape Explained.
From www.youtube.com
Acid Base Titration Curves Simplified YouTube Titration Curve Shape Explained Everything you need to know for a. In the examples shown below, alkali is added to an acid. a summary of the important curves. the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what. On the left is a titration in which the base is. Titration Curve Shape Explained.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation, free Titration Curve Shape Explained A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. a summary of the important curves. how do you explain the shape of a titration curve? As the starting solution is. a titration curve is a graphical representation of the ph of a solution during a titration. In the examples. Titration Curve Shape Explained.
From kwokthechemteacher.blogspot.sg
KWOK The Chem Teacher ionic equilibrium titration curves Titration Curve Shape Explained a summary of the important curves. a titration curve is a graphical representation of the ph of a solution during a titration. Everything you need to know for a. And why is the equivalence point not always at ph7? On the left is a titration in which the base is added to the acid, and so the ph. Titration Curve Shape Explained.
From www.slideserve.com
PPT Shape of titration curve… PowerPoint Presentation, free download Titration Curve Shape Explained a titration curve is a graphical representation of the ph of a solution during a titration. there are four shapes of titration curves that you need to be able to explain. In the examples shown below, alkali is added to an acid. The way you normally carry out a titration involves adding the acid to the alkali. . Titration Curve Shape Explained.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Titration Curve Shape Explained how do you explain the shape of a titration curve? the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what. there are four shapes of titration curves that you need to be able to explain. On the left is a titration in which the. Titration Curve Shape Explained.
From www.youtube.com
Intro to Titration Curves and the Important Parts YouTube Titration Curve Shape Explained a summary of the important curves. a titration curve is a graphical representation of the ph of a solution during a titration. how do you explain the shape of a titration curve? A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. Everything you need to know for a. . Titration Curve Shape Explained.
From www.youtube.com
Shapes of Titration Curves YouTube Titration Curve Shape Explained the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what. a titration curve is a graphical representation of the ph of a solution during a titration. a summary of the important curves. there are four shapes of titration curves that you need to. Titration Curve Shape Explained.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Curve Shape Explained On the left is a titration in which the base is added to the acid, and so the ph progresses from low to high. As the starting solution is. a titration curve is a graphical representation of the ph of a solution during a titration. The figure below shows two different. And why is the equivalence point not always. Titration Curve Shape Explained.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration Curve Shape Explained A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. a titration curve is a graphical representation of the ph of a solution during a titration. In the examples shown below, alkali is added to an acid. a summary of the important curves. On the left is a titration in which. Titration Curve Shape Explained.
From crunchchemistry.co.uk
How to explain the shape of a titration curve Crunch Chemistry Titration Curve Shape Explained the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what. And why is the equivalence point not always at ph7? a summary of the important curves. there are four shapes of titration curves that you need to be able to explain. As the starting. Titration Curve Shape Explained.
From fernando-well-key.blogspot.com
How to Describe a Titration Curve Titration Curve Shape Explained a titration curve is a graphical representation of the ph of a solution during a titration. how do you explain the shape of a titration curve? The way you normally carry out a titration involves adding the acid to the alkali. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide.. Titration Curve Shape Explained.
From www.showme.com
Titration Curve Explained Science, Chemistry ShowMe Titration Curve Shape Explained The figure below shows two different. As the starting solution is. In the examples shown below, alkali is added to an acid. there are four shapes of titration curves that you need to be able to explain. a titration curve is a graphical representation of the ph of a solution during a titration. A typical titration curve of. Titration Curve Shape Explained.
From quizlet.com
Explain what a titration curve is, and sketch its shape. Quizlet Titration Curve Shape Explained The way you normally carry out a titration involves adding the acid to the alkali. And why is the equivalence point not always at ph7? a titration curve is a graphical representation of the ph of a solution during a titration. there are four shapes of titration curves that you need to be able to explain. In the. Titration Curve Shape Explained.
From exokpafwp.blob.core.windows.net
Titration Endpoint Calculator at Kevin Dowell blog Titration Curve Shape Explained a titration curve is a graphical representation of the ph of a solution during a titration. As the starting solution is. Everything you need to know for a. there are four shapes of titration curves that you need to be able to explain. a summary of the important curves. The way you normally carry out a titration. Titration Curve Shape Explained.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Curve Shape Explained The figure below shows two different. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. a titration curve is a graphical representation of the ph of a solution during a titration. how do you explain the shape of a titration curve? Everything you need to know for a. a. Titration Curve Shape Explained.
From mungfali.com
Acid Titration Curve Titration Curve Shape Explained how do you explain the shape of a titration curve? And why is the equivalence point not always at ph7? a summary of the important curves. there are four shapes of titration curves that you need to be able to explain. In the examples shown below, alkali is added to an acid. As the starting solution is.. Titration Curve Shape Explained.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps Titration Curve Shape Explained The figure below shows two different. Everything you need to know for a. The way you normally carry out a titration involves adding the acid to the alkali. As the starting solution is. In the examples shown below, alkali is added to an acid. a titration curve is a graphical representation of the ph of a solution during a. Titration Curve Shape Explained.
From www.youtube.com
Explaining the shape of titration curves YouTube Titration Curve Shape Explained a titration curve is a graphical representation of the ph of a solution during a titration. Everything you need to know for a. In the examples shown below, alkali is added to an acid. On the left is a titration in which the base is added to the acid, and so the ph progresses from low to high. . Titration Curve Shape Explained.
From mungfali.com
Titration Curve Labeled Titration Curve Shape Explained there are four shapes of titration curves that you need to be able to explain. And why is the equivalence point not always at ph7? the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what. a summary of the important curves. The figure below. Titration Curve Shape Explained.
From aracely-has-zhang.blogspot.com
How to Describe a Titration Curve AracelyhasZhang Titration Curve Shape Explained how do you explain the shape of a titration curve? On the left is a titration in which the base is added to the acid, and so the ph progresses from low to high. a summary of the important curves. a titration curve is a graphical representation of the ph of a solution during a titration. . Titration Curve Shape Explained.
From crunchchemistry.co.uk
How to explain the shape of a titration curve Crunch Chemistry Titration Curve Shape Explained there are four shapes of titration curves that you need to be able to explain. In the examples shown below, alkali is added to an acid. the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what. The figure below shows two different. A typical titration. Titration Curve Shape Explained.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration Curve Shape Explained As the starting solution is. how do you explain the shape of a titration curve? The figure below shows two different. On the left is a titration in which the base is added to the acid, and so the ph progresses from low to high. The way you normally carry out a titration involves adding the acid to the. Titration Curve Shape Explained.
From www.slideserve.com
PPT Titration Curves PowerPoint Presentation, free download ID3970559 Titration Curve Shape Explained The way you normally carry out a titration involves adding the acid to the alkali. On the left is a titration in which the base is added to the acid, and so the ph progresses from low to high. there are four shapes of titration curves that you need to be able to explain. A typical titration curve of. Titration Curve Shape Explained.
From glossary.periodni.com
Titration curve Chemistry Dictionary & Glossary Titration Curve Shape Explained Everything you need to know for a. a titration curve is a graphical representation of the ph of a solution during a titration. how do you explain the shape of a titration curve? there are four shapes of titration curves that you need to be able to explain. In the examples shown below, alkali is added to. Titration Curve Shape Explained.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Titration Curve Shape Explained a titration curve is a graphical representation of the ph of a solution during a titration. In the examples shown below, alkali is added to an acid. Everything you need to know for a. the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what. On. Titration Curve Shape Explained.
From webmis.highland.cc.il.us
AcidBase Titrations Titration Curve Shape Explained the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what. And why is the equivalence point not always at ph7? On the left is a titration in which the base is added to the acid, and so the ph progresses from low to high. a. Titration Curve Shape Explained.
From www.youtube.com
Titration Curves for High School Chemistry YouTube Titration Curve Shape Explained The figure below shows two different. On the left is a titration in which the base is added to the acid, and so the ph progresses from low to high. a summary of the important curves. a titration curve is a graphical representation of the ph of a solution during a titration. a titration curve is a. Titration Curve Shape Explained.
From generalchemistrylab.blogspot.com
Chemistry Laboratory Titration curve & HendersonHasselbalch equation Titration Curve Shape Explained The way you normally carry out a titration involves adding the acid to the alkali. In the examples shown below, alkali is added to an acid. And why is the equivalence point not always at ph7? The figure below shows two different. a titration curve is a graphical representation of the ph of a solution during a titration. . Titration Curve Shape Explained.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Titration Curve Shape Explained a summary of the important curves. there are four shapes of titration curves that you need to be able to explain. The way you normally carry out a titration involves adding the acid to the alkali. a titration curve is a graphical representation of the ph of a solution during a titration. a titration curve is. Titration Curve Shape Explained.
From www.youtube.com
Acid Base Titration Curves YouTube Titration Curve Shape Explained a titration curve is a graphical representation of the ph of a solution during a titration. As the starting solution is. a summary of the important curves. a titration curve is a graphical representation of the ph of a solution during a titration. there are four shapes of titration curves that you need to be able. Titration Curve Shape Explained.
From www.youtube.com
Amino Acids Titration Curves Explained YouTube Titration Curve Shape Explained The figure below shows two different. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. In the examples shown below, alkali is added to an acid. the shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what. On the. Titration Curve Shape Explained.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Titration Curve Shape Explained And why is the equivalence point not always at ph7? a titration curve is a graphical representation of the ph of a solution during a titration. The way you normally carry out a titration involves adding the acid to the alkali. how do you explain the shape of a titration curve? On the left is a titration in. Titration Curve Shape Explained.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Titration Curve Shape Explained a titration curve is a graphical representation of the ph of a solution during a titration. how do you explain the shape of a titration curve? Everything you need to know for a. As the starting solution is. In the examples shown below, alkali is added to an acid. And why is the equivalence point not always at. Titration Curve Shape Explained.
From www.scribd.com
Explaining The Shape of Titration Curves PDF Acid Hydroxide Titration Curve Shape Explained On the left is a titration in which the base is added to the acid, and so the ph progresses from low to high. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. a titration curve is a graphical representation of the ph of a solution during a titration. And why. Titration Curve Shape Explained.