Colorimetry Results For Copper Sulphate . this investigation shows you how to use a sciencescope. the primary objective of this experiment is to determine the concentration of an unknown copper (ii) sulfate solution. colorimetry is a mainly quantitative technique that is used to determine the concentration of solute particles in a. to determine the wavelength (color) of maximum absorbance for a copper (ii) sulfate solution. To examine the relationship between the absorbance. Of the four wavelengths available on the colorimeter (430 nm, 470 nm, 565 nm, and 635 nm), the. The cuso 4 solution used in this experiment has a blue color, so colorimeter users will be instructed to use the red led. The objective of our colourimetry experiment is to determine the unknown concentration of copper sulfate (cuso 4). the results of the experiment. 10k views 3 years ago. This video explains the experimental.
from studylib.net
this investigation shows you how to use a sciencescope. Of the four wavelengths available on the colorimeter (430 nm, 470 nm, 565 nm, and 635 nm), the. This video explains the experimental. To examine the relationship between the absorbance. the primary objective of this experiment is to determine the concentration of an unknown copper (ii) sulfate solution. colorimetry is a mainly quantitative technique that is used to determine the concentration of solute particles in a. the results of the experiment. The cuso 4 solution used in this experiment has a blue color, so colorimeter users will be instructed to use the red led. 10k views 3 years ago. The objective of our colourimetry experiment is to determine the unknown concentration of copper sulfate (cuso 4).
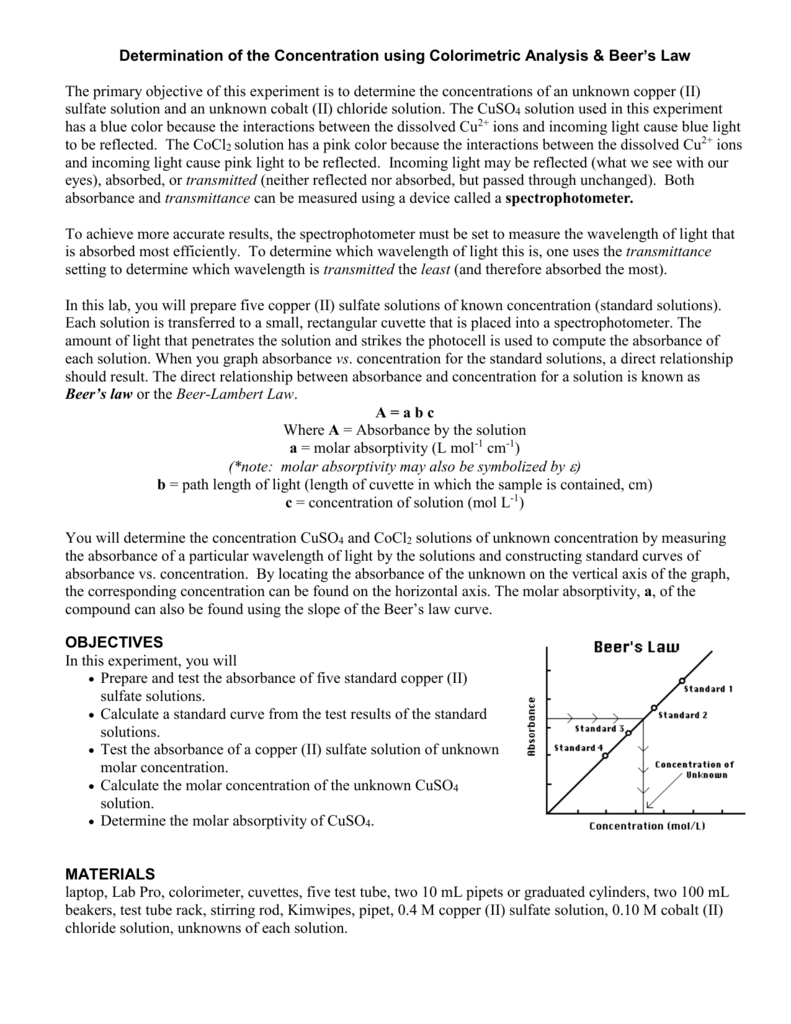
Determination of Concentration using Colorimetric Analysis & Beer`s
Colorimetry Results For Copper Sulphate Of the four wavelengths available on the colorimeter (430 nm, 470 nm, 565 nm, and 635 nm), the. This video explains the experimental. the primary objective of this experiment is to determine the concentration of an unknown copper (ii) sulfate solution. The cuso 4 solution used in this experiment has a blue color, so colorimeter users will be instructed to use the red led. this investigation shows you how to use a sciencescope. The objective of our colourimetry experiment is to determine the unknown concentration of copper sulfate (cuso 4). to determine the wavelength (color) of maximum absorbance for a copper (ii) sulfate solution. Of the four wavelengths available on the colorimeter (430 nm, 470 nm, 565 nm, and 635 nm), the. To examine the relationship between the absorbance. the results of the experiment. colorimetry is a mainly quantitative technique that is used to determine the concentration of solute particles in a. 10k views 3 years ago.
From www.animalia-life.club
Crystallisation Of Copper Sulphate Colorimetry Results For Copper Sulphate The objective of our colourimetry experiment is to determine the unknown concentration of copper sulfate (cuso 4). 10k views 3 years ago. to determine the wavelength (color) of maximum absorbance for a copper (ii) sulfate solution. this investigation shows you how to use a sciencescope. This video explains the experimental. the primary objective of this experiment is. Colorimetry Results For Copper Sulphate.
From www.youtube.com
Determination of Copper in Brass YouTube Colorimetry Results For Copper Sulphate to determine the wavelength (color) of maximum absorbance for a copper (ii) sulfate solution. colorimetry is a mainly quantitative technique that is used to determine the concentration of solute particles in a. the primary objective of this experiment is to determine the concentration of an unknown copper (ii) sulfate solution. To examine the relationship between the absorbance.. Colorimetry Results For Copper Sulphate.
From www.youtube.com
Colorimetric estimation of copper YouTube Colorimetry Results For Copper Sulphate Of the four wavelengths available on the colorimeter (430 nm, 470 nm, 565 nm, and 635 nm), the. 10k views 3 years ago. the results of the experiment. colorimetry is a mainly quantitative technique that is used to determine the concentration of solute particles in a. This video explains the experimental. The objective of our colourimetry experiment is. Colorimetry Results For Copper Sulphate.
From www.researchgate.net
Effect of different concentrations of copper sulphate (g kg1 soil Colorimetry Results For Copper Sulphate 10k views 3 years ago. This video explains the experimental. this investigation shows you how to use a sciencescope. colorimetry is a mainly quantitative technique that is used to determine the concentration of solute particles in a. The objective of our colourimetry experiment is to determine the unknown concentration of copper sulfate (cuso 4). the primary objective. Colorimetry Results For Copper Sulphate.
From www.odinity.com
Determination of Cupric Sulfate Hydration Number Odinity Colorimetry Results For Copper Sulphate colorimetry is a mainly quantitative technique that is used to determine the concentration of solute particles in a. The cuso 4 solution used in this experiment has a blue color, so colorimeter users will be instructed to use the red led. the primary objective of this experiment is to determine the concentration of an unknown copper (ii) sulfate. Colorimetry Results For Copper Sulphate.
From www.youtube.com
Exp 7 Quantitative Determination of Copper (II) Sulfate Concentration Colorimetry Results For Copper Sulphate this investigation shows you how to use a sciencescope. To examine the relationship between the absorbance. The cuso 4 solution used in this experiment has a blue color, so colorimeter users will be instructed to use the red led. to determine the wavelength (color) of maximum absorbance for a copper (ii) sulfate solution. 10k views 3 years ago.. Colorimetry Results For Copper Sulphate.
From www.echemi.com
Molar Absorptivity of Copper(II) Sulfate ECHEMI Colorimetry Results For Copper Sulphate to determine the wavelength (color) of maximum absorbance for a copper (ii) sulfate solution. Of the four wavelengths available on the colorimeter (430 nm, 470 nm, 565 nm, and 635 nm), the. The cuso 4 solution used in this experiment has a blue color, so colorimeter users will be instructed to use the red led. The objective of our. Colorimetry Results For Copper Sulphate.
From www.youtube.com
Spectrophotometer Beer's Law And Colorimetry Lab YouTube Colorimetry Results For Copper Sulphate the primary objective of this experiment is to determine the concentration of an unknown copper (ii) sulfate solution. The cuso 4 solution used in this experiment has a blue color, so colorimeter users will be instructed to use the red led. this investigation shows you how to use a sciencescope. This video explains the experimental. To examine the. Colorimetry Results For Copper Sulphate.
From www.youtube.com
Colorimetric estimation of Copper YouTube Colorimetry Results For Copper Sulphate 10k views 3 years ago. Of the four wavelengths available on the colorimeter (430 nm, 470 nm, 565 nm, and 635 nm), the. colorimetry is a mainly quantitative technique that is used to determine the concentration of solute particles in a. this investigation shows you how to use a sciencescope. The objective of our colourimetry experiment is to. Colorimetry Results For Copper Sulphate.
From www.orientjchem.org
Thermodynamic study of copper sulphate and zinc sulphate in water and Colorimetry Results For Copper Sulphate the primary objective of this experiment is to determine the concentration of an unknown copper (ii) sulfate solution. the results of the experiment. Of the four wavelengths available on the colorimeter (430 nm, 470 nm, 565 nm, and 635 nm), the. to determine the wavelength (color) of maximum absorbance for a copper (ii) sulfate solution. colorimetry. Colorimetry Results For Copper Sulphate.
From www.youtube.com
Analyzing a Copper Solution Using Colorimetry YouTube Colorimetry Results For Copper Sulphate The cuso 4 solution used in this experiment has a blue color, so colorimeter users will be instructed to use the red led. the primary objective of this experiment is to determine the concentration of an unknown copper (ii) sulfate solution. to determine the wavelength (color) of maximum absorbance for a copper (ii) sulfate solution. the results. Colorimetry Results For Copper Sulphate.
From studymoose.com
Colorimetry Experiment Determining Copper Sulfate Concentration Colorimetry Results For Copper Sulphate This video explains the experimental. this investigation shows you how to use a sciencescope. colorimetry is a mainly quantitative technique that is used to determine the concentration of solute particles in a. The objective of our colourimetry experiment is to determine the unknown concentration of copper sulfate (cuso 4). The cuso 4 solution used in this experiment has. Colorimetry Results For Copper Sulphate.
From philippinejournalofpathology.org
The Effect of Number of Tests, Hemoglobin Level and Working Temperature Colorimetry Results For Copper Sulphate Of the four wavelengths available on the colorimeter (430 nm, 470 nm, 565 nm, and 635 nm), the. colorimetry is a mainly quantitative technique that is used to determine the concentration of solute particles in a. The cuso 4 solution used in this experiment has a blue color, so colorimeter users will be instructed to use the red led.. Colorimetry Results For Copper Sulphate.
From www.researchgate.net
Fig. S10 UVVis spectrum of copper(II) sulfate. Download Scientific Colorimetry Results For Copper Sulphate The objective of our colourimetry experiment is to determine the unknown concentration of copper sulfate (cuso 4). to determine the wavelength (color) of maximum absorbance for a copper (ii) sulfate solution. colorimetry is a mainly quantitative technique that is used to determine the concentration of solute particles in a. the primary objective of this experiment is to. Colorimetry Results For Copper Sulphate.
From www.youtube.com
Demonstration of the experiment Estimation of copper by Colorimetric Colorimetry Results For Copper Sulphate This video explains the experimental. Of the four wavelengths available on the colorimeter (430 nm, 470 nm, 565 nm, and 635 nm), the. 10k views 3 years ago. the results of the experiment. this investigation shows you how to use a sciencescope. to determine the wavelength (color) of maximum absorbance for a copper (ii) sulfate solution. The. Colorimetry Results For Copper Sulphate.
From www.researchgate.net
Absorbance spectra of the dye/copper sulfate system at pH 10.7 and Colorimetry Results For Copper Sulphate This video explains the experimental. this investigation shows you how to use a sciencescope. the results of the experiment. colorimetry is a mainly quantitative technique that is used to determine the concentration of solute particles in a. the primary objective of this experiment is to determine the concentration of an unknown copper (ii) sulfate solution. Of. Colorimetry Results For Copper Sulphate.
From www.intechopen.com
Colorimetric Detection of Copper Ion Based on Click Chemistry IntechOpen Colorimetry Results For Copper Sulphate The objective of our colourimetry experiment is to determine the unknown concentration of copper sulfate (cuso 4). This video explains the experimental. The cuso 4 solution used in this experiment has a blue color, so colorimeter users will be instructed to use the red led. 10k views 3 years ago. to determine the wavelength (color) of maximum absorbance for. Colorimetry Results For Copper Sulphate.
From dxodluoya.blob.core.windows.net
Colorimetry Uses In Industry at Laura Clay blog Colorimetry Results For Copper Sulphate the results of the experiment. To examine the relationship between the absorbance. this investigation shows you how to use a sciencescope. colorimetry is a mainly quantitative technique that is used to determine the concentration of solute particles in a. Of the four wavelengths available on the colorimeter (430 nm, 470 nm, 565 nm, and 635 nm), the.. Colorimetry Results For Copper Sulphate.
From www.arigobio.com
Copper Assay Kit (Colorimetric) (ARG82148) arigo Biolaboratories Colorimetry Results For Copper Sulphate colorimetry is a mainly quantitative technique that is used to determine the concentration of solute particles in a. To examine the relationship between the absorbance. this investigation shows you how to use a sciencescope. The objective of our colourimetry experiment is to determine the unknown concentration of copper sulfate (cuso 4). to determine the wavelength (color) of. Colorimetry Results For Copper Sulphate.
From www.elabscience.cn
ebck300m Elabscience Colorimetry Results For Copper Sulphate The objective of our colourimetry experiment is to determine the unknown concentration of copper sulfate (cuso 4). to determine the wavelength (color) of maximum absorbance for a copper (ii) sulfate solution. This video explains the experimental. Of the four wavelengths available on the colorimeter (430 nm, 470 nm, 565 nm, and 635 nm), the. The cuso 4 solution used. Colorimetry Results For Copper Sulphate.
From www.youtube.com
Colorimetric estimation of copper YouTube Colorimetry Results For Copper Sulphate 10k views 3 years ago. This video explains the experimental. the primary objective of this experiment is to determine the concentration of an unknown copper (ii) sulfate solution. the results of the experiment. To examine the relationship between the absorbance. The objective of our colourimetry experiment is to determine the unknown concentration of copper sulfate (cuso 4). The. Colorimetry Results For Copper Sulphate.
From www.scribd.com
Experiment 12 Results and Discussion Report Determination of Copper Colorimetry Results For Copper Sulphate the primary objective of this experiment is to determine the concentration of an unknown copper (ii) sulfate solution. To examine the relationship between the absorbance. colorimetry is a mainly quantitative technique that is used to determine the concentration of solute particles in a. this investigation shows you how to use a sciencescope. Of the four wavelengths available. Colorimetry Results For Copper Sulphate.
From www.youtube.com
To determine the concentration of given copper sulphate solution using Colorimetry Results For Copper Sulphate To examine the relationship between the absorbance. 10k views 3 years ago. The objective of our colourimetry experiment is to determine the unknown concentration of copper sulfate (cuso 4). the primary objective of this experiment is to determine the concentration of an unknown copper (ii) sulfate solution. This video explains the experimental. the results of the experiment. . Colorimetry Results For Copper Sulphate.
From www.youtube.com
observations copper II sulphate and water by simple distillation YouTube Colorimetry Results For Copper Sulphate colorimetry is a mainly quantitative technique that is used to determine the concentration of solute particles in a. Of the four wavelengths available on the colorimeter (430 nm, 470 nm, 565 nm, and 635 nm), the. The cuso 4 solution used in this experiment has a blue color, so colorimeter users will be instructed to use the red led.. Colorimetry Results For Copper Sulphate.
From www.youtube.com
Heating Hydrated Copper Sulphate CuSO4.5H2O Water of crystallization Colorimetry Results For Copper Sulphate the results of the experiment. Of the four wavelengths available on the colorimeter (430 nm, 470 nm, 565 nm, and 635 nm), the. colorimetry is a mainly quantitative technique that is used to determine the concentration of solute particles in a. this investigation shows you how to use a sciencescope. This video explains the experimental. The cuso. Colorimetry Results For Copper Sulphate.
From microbenotes.com
Colorimeter Definition, Principle, Parts, Uses, Examples Colorimetry Results For Copper Sulphate The cuso 4 solution used in this experiment has a blue color, so colorimeter users will be instructed to use the red led. the results of the experiment. the primary objective of this experiment is to determine the concentration of an unknown copper (ii) sulfate solution. To examine the relationship between the absorbance. The objective of our colourimetry. Colorimetry Results For Copper Sulphate.
From www.youtube.com
Colorimetric Estimation of Copper Sulphate YouTube Colorimetry Results For Copper Sulphate this investigation shows you how to use a sciencescope. To examine the relationship between the absorbance. the results of the experiment. colorimetry is a mainly quantitative technique that is used to determine the concentration of solute particles in a. Of the four wavelengths available on the colorimeter (430 nm, 470 nm, 565 nm, and 635 nm), the.. Colorimetry Results For Copper Sulphate.
From studylib.net
Determination of Concentration using Colorimetric Analysis & Beer`s Colorimetry Results For Copper Sulphate This video explains the experimental. The cuso 4 solution used in this experiment has a blue color, so colorimeter users will be instructed to use the red led. 10k views 3 years ago. the results of the experiment. this investigation shows you how to use a sciencescope. To examine the relationship between the absorbance. The objective of our. Colorimetry Results For Copper Sulphate.
From pixels.com
Copper Sulphate Reacts With Ammonium Photograph by Jerry Mason/science Colorimetry Results For Copper Sulphate to determine the wavelength (color) of maximum absorbance for a copper (ii) sulfate solution. This video explains the experimental. Of the four wavelengths available on the colorimeter (430 nm, 470 nm, 565 nm, and 635 nm), the. 10k views 3 years ago. The cuso 4 solution used in this experiment has a blue color, so colorimeter users will be. Colorimetry Results For Copper Sulphate.
From www.semanticscholar.org
Figure 1 from A rapid colorimetric method for the quantitative Colorimetry Results For Copper Sulphate the results of the experiment. This video explains the experimental. this investigation shows you how to use a sciencescope. to determine the wavelength (color) of maximum absorbance for a copper (ii) sulfate solution. The objective of our colourimetry experiment is to determine the unknown concentration of copper sulfate (cuso 4). The cuso 4 solution used in this. Colorimetry Results For Copper Sulphate.
From www.researchgate.net
Effect of copper sulphate concentration on the colour strength of dyed Colorimetry Results For Copper Sulphate the results of the experiment. colorimetry is a mainly quantitative technique that is used to determine the concentration of solute particles in a. 10k views 3 years ago. The objective of our colourimetry experiment is to determine the unknown concentration of copper sulfate (cuso 4). the primary objective of this experiment is to determine the concentration of. Colorimetry Results For Copper Sulphate.
From www.alamy.com
Student using a colorimeter to measure the hue of a sample of copper Colorimetry Results For Copper Sulphate the results of the experiment. colorimetry is a mainly quantitative technique that is used to determine the concentration of solute particles in a. To examine the relationship between the absorbance. 10k views 3 years ago. this investigation shows you how to use a sciencescope. to determine the wavelength (color) of maximum absorbance for a copper (ii). Colorimetry Results For Copper Sulphate.
From www.alamy.com
Copper sulphate formation. Test tube of copper (II) sulphate (blue) and Colorimetry Results For Copper Sulphate Of the four wavelengths available on the colorimeter (430 nm, 470 nm, 565 nm, and 635 nm), the. colorimetry is a mainly quantitative technique that is used to determine the concentration of solute particles in a. To examine the relationship between the absorbance. This video explains the experimental. to determine the wavelength (color) of maximum absorbance for a. Colorimetry Results For Copper Sulphate.
From www.slideserve.com
PPT Colorimetry PowerPoint Presentation ID2509705 Colorimetry Results For Copper Sulphate This video explains the experimental. The objective of our colourimetry experiment is to determine the unknown concentration of copper sulfate (cuso 4). To examine the relationship between the absorbance. to determine the wavelength (color) of maximum absorbance for a copper (ii) sulfate solution. Of the four wavelengths available on the colorimeter (430 nm, 470 nm, 565 nm, and 635. Colorimetry Results For Copper Sulphate.
From www.researchgate.net
Calibration curve for copper(II) concentration vs absorbance Colorimetry Results For Copper Sulphate the primary objective of this experiment is to determine the concentration of an unknown copper (ii) sulfate solution. The objective of our colourimetry experiment is to determine the unknown concentration of copper sulfate (cuso 4). 10k views 3 years ago. Of the four wavelengths available on the colorimeter (430 nm, 470 nm, 565 nm, and 635 nm), the. . Colorimetry Results For Copper Sulphate.