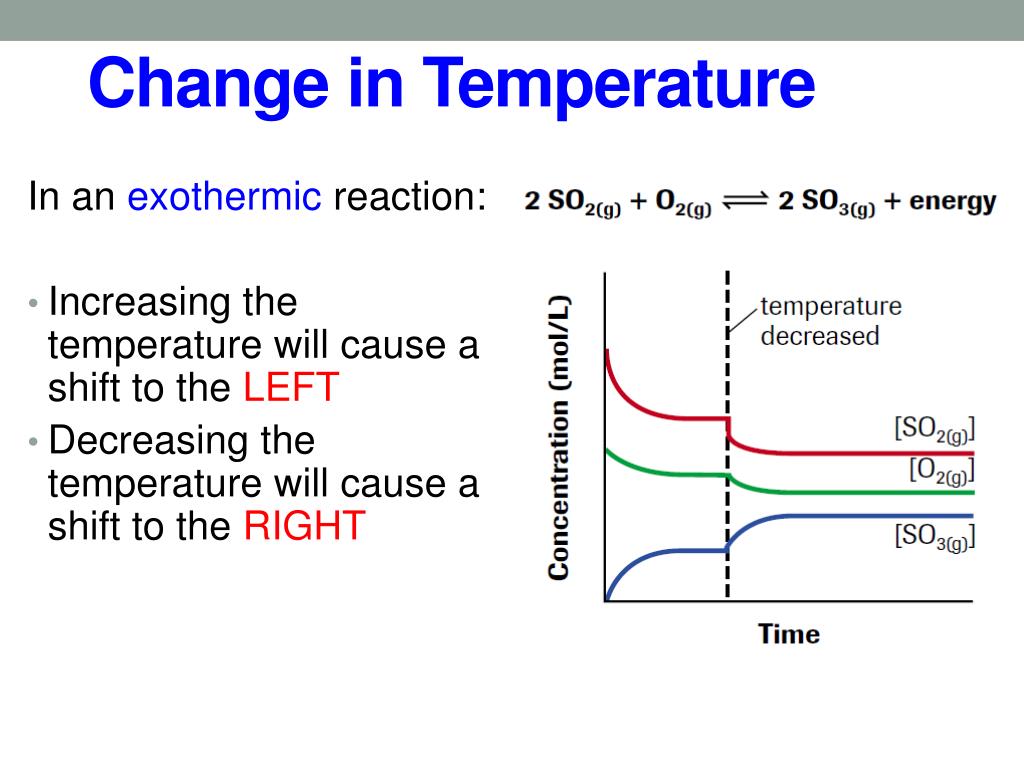
Endothermic Reaction Temperature Decrease . in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings. a temperature change occurs when temperature is increased or decreased by the flow of heat. in an endothermic change, temperature is absorbed from surrounding molecules to continue reacting. Some examples of endothermic reactions are: (6 answers) closed 3 years ago. there are two methods for distinguishing between exothermic and endothermic reactions. how can decreasing in temperature indicate an endothermic reaction? When energy is released in an. an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. a thermometer is used to detect the temperature decrease.
from www.slideserve.com
When energy is released in an. how can decreasing in temperature indicate an endothermic reaction? a temperature change occurs when temperature is increased or decreased by the flow of heat. an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings. (6 answers) closed 3 years ago. a thermometer is used to detect the temperature decrease. there are two methods for distinguishing between exothermic and endothermic reactions. in an endothermic change, temperature is absorbed from surrounding molecules to continue reacting. Some examples of endothermic reactions are:
PPT Le Chatelier’s Principle PowerPoint Presentation, free download
Endothermic Reaction Temperature Decrease an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. in an endothermic change, temperature is absorbed from surrounding molecules to continue reacting. When energy is released in an. in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings. how can decreasing in temperature indicate an endothermic reaction? Some examples of endothermic reactions are: (6 answers) closed 3 years ago. there are two methods for distinguishing between exothermic and endothermic reactions. an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. a temperature change occurs when temperature is increased or decreased by the flow of heat. a thermometer is used to detect the temperature decrease.
From classnotes123.com
What does one mean by exothermic and endothermic reactions? Give Endothermic Reaction Temperature Decrease in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings. how can decreasing in temperature indicate an endothermic reaction? an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. there are two methods for distinguishing between exothermic and endothermic reactions.. Endothermic Reaction Temperature Decrease.
From eduinput.com
Differences Between Endotherm And Ectotherm Endothermic Reaction Temperature Decrease a temperature change occurs when temperature is increased or decreased by the flow of heat. in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings. (6 answers) closed 3 years ago. there are two methods for distinguishing between exothermic and endothermic reactions. When energy is released. Endothermic Reaction Temperature Decrease.
From khatiar1955a.altervista.org
The Difference Between Endothermic and Exothermic Reactions Endothermic Reaction Temperature Decrease Some examples of endothermic reactions are: there are two methods for distinguishing between exothermic and endothermic reactions. in an endothermic change, temperature is absorbed from surrounding molecules to continue reacting. When energy is released in an. how can decreasing in temperature indicate an endothermic reaction? a thermometer is used to detect the temperature decrease. a. Endothermic Reaction Temperature Decrease.
From ar.inspiredpencil.com
Endothermic And Exothermic Reactions Temperature Change Endothermic Reaction Temperature Decrease (6 answers) closed 3 years ago. When energy is released in an. an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings. in an endothermic change, temperature is absorbed from surrounding. Endothermic Reaction Temperature Decrease.
From www.studyorgo.com
How to Interpret Thermodynamics of Reactions Endothermic Reaction Temperature Decrease an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. there are two methods for distinguishing between exothermic and endothermic reactions. (6 answers) closed 3 years ago. Some examples of endothermic reactions are: in an endothermic change, temperature is absorbed from surrounding molecules to continue reacting. how can decreasing in temperature. Endothermic Reaction Temperature Decrease.
From www.numerade.com
SOLVEDAn endothermic reaction causes the surroundings to condense Endothermic Reaction Temperature Decrease how can decreasing in temperature indicate an endothermic reaction? a thermometer is used to detect the temperature decrease. an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. in an endothermic change, temperature is absorbed from surrounding molecules to continue reacting. there are two methods for distinguishing between exothermic and. Endothermic Reaction Temperature Decrease.
From h-o-m-e.org
Endothermic Reactions The Science Behind Temperature Change Endothermic Reaction Temperature Decrease an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. a temperature change occurs when temperature is increased or decreased by the flow of heat. Some examples of endothermic reactions are: When energy is released in an. (6 answers) closed 3 years ago. how can decreasing in temperature indicate an endothermic reaction?. Endothermic Reaction Temperature Decrease.
From ar.inspiredpencil.com
Endothermic And Exothermic Reactions Temperature Change Endothermic Reaction Temperature Decrease When energy is released in an. (6 answers) closed 3 years ago. an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. in an endothermic change, temperature is absorbed from surrounding molecules to continue reacting. a temperature change occurs when temperature is increased or decreased by the flow of heat. Some examples. Endothermic Reaction Temperature Decrease.
From en.ppt-online.org
Thermal Energy, Chemical Energy online presentation Endothermic Reaction Temperature Decrease When energy is released in an. there are two methods for distinguishing between exothermic and endothermic reactions. in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings. an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. a temperature change. Endothermic Reaction Temperature Decrease.
From www.w3schools.blog
Factors affecting the rate of a reaction Temperature W3schools Endothermic Reaction Temperature Decrease (6 answers) closed 3 years ago. a temperature change occurs when temperature is increased or decreased by the flow of heat. in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings. how can decreasing in temperature indicate an endothermic reaction? in an endothermic change, temperature. Endothermic Reaction Temperature Decrease.
From ar.inspiredpencil.com
Endothermic And Exothermic Reactions Temperature Change Endothermic Reaction Temperature Decrease in an endothermic change, temperature is absorbed from surrounding molecules to continue reacting. (6 answers) closed 3 years ago. Some examples of endothermic reactions are: in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings. When energy is released in an. how can decreasing in temperature. Endothermic Reaction Temperature Decrease.
From www.chemistrystudent.com
Equilibrium (ALevel) ChemistryStudent Endothermic Reaction Temperature Decrease an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. how can decreasing in temperature indicate an endothermic reaction? in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings. Some examples of endothermic reactions are: (6 answers) closed 3 years ago.. Endothermic Reaction Temperature Decrease.
From proper-cooking.info
Endothermic And Exothermic Reactions Temperature Change Endothermic Reaction Temperature Decrease a thermometer is used to detect the temperature decrease. there are two methods for distinguishing between exothermic and endothermic reactions. (6 answers) closed 3 years ago. a temperature change occurs when temperature is increased or decreased by the flow of heat. an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings.. Endothermic Reaction Temperature Decrease.
From www.numerade.com
SOLVED An endothermIc reaction causes the surroundings to Multlple Endothermic Reaction Temperature Decrease a thermometer is used to detect the temperature decrease. Some examples of endothermic reactions are: an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings. a temperature change occurs when. Endothermic Reaction Temperature Decrease.
From www.slideserve.com
PPT Le Chatelier’s Principle PowerPoint Presentation, free download Endothermic Reaction Temperature Decrease (6 answers) closed 3 years ago. When energy is released in an. Some examples of endothermic reactions are: a temperature change occurs when temperature is increased or decreased by the flow of heat. how can decreasing in temperature indicate an endothermic reaction? in the course of an endothermic process, the system gains heat from the surroundings and. Endothermic Reaction Temperature Decrease.
From wiredataremetev5.z4.web.core.windows.net
Endothermic And Exothermic Reaction Diagram Endothermic Reaction Temperature Decrease in an endothermic change, temperature is absorbed from surrounding molecules to continue reacting. in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings. (6 answers) closed 3 years ago. When energy is released in an. Some examples of endothermic reactions are: a thermometer is used to. Endothermic Reaction Temperature Decrease.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps Endothermic Reaction Temperature Decrease When energy is released in an. in an endothermic change, temperature is absorbed from surrounding molecules to continue reacting. Some examples of endothermic reactions are: in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings. a temperature change occurs when temperature is increased or decreased by. Endothermic Reaction Temperature Decrease.
From sciencenotes.org
Le Chatelier's Principle Endothermic Reaction Temperature Decrease in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings. a thermometer is used to detect the temperature decrease. there are two methods for distinguishing between exothermic and endothermic reactions. a temperature change occurs when temperature is increased or decreased by the flow of heat.. Endothermic Reaction Temperature Decrease.
From slidetodoc.com
Chemical Equilibrium Chapter 14 Chemical Equilibrium 14 1 Endothermic Reaction Temperature Decrease how can decreasing in temperature indicate an endothermic reaction? When energy is released in an. in an endothermic change, temperature is absorbed from surrounding molecules to continue reacting. there are two methods for distinguishing between exothermic and endothermic reactions. Some examples of endothermic reactions are: an endothermic reaction occurs when the temperature of an isolated system. Endothermic Reaction Temperature Decrease.
From www.chegg.com
Solved Question 17CO2+H2⇌CO+H2OIncreasing the temperature of Endothermic Reaction Temperature Decrease how can decreasing in temperature indicate an endothermic reaction? Some examples of endothermic reactions are: When energy is released in an. a temperature change occurs when temperature is increased or decreased by the flow of heat. (6 answers) closed 3 years ago. an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings.. Endothermic Reaction Temperature Decrease.
From www.expii.com
Spontaneous and Nonspontaneous Reactions — Overview Expii Endothermic Reaction Temperature Decrease how can decreasing in temperature indicate an endothermic reaction? in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings. a thermometer is used to detect the temperature decrease. (6 answers) closed 3 years ago. a temperature change occurs when temperature is increased or decreased by. Endothermic Reaction Temperature Decrease.
From askfilo.com
The change in temperature noticed i. endothermic reaction ii. the reactio.. Endothermic Reaction Temperature Decrease When energy is released in an. in an endothermic change, temperature is absorbed from surrounding molecules to continue reacting. (6 answers) closed 3 years ago. a thermometer is used to detect the temperature decrease. Some examples of endothermic reactions are: a temperature change occurs when temperature is increased or decreased by the flow of heat. in. Endothermic Reaction Temperature Decrease.
From schematiclistmotus123.z13.web.core.windows.net
Enthalpy Profile Diagram Endothermic Reaction Endothermic Reaction Temperature Decrease an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. a thermometer is used to detect the temperature decrease. Some examples of endothermic reactions are: in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings. a temperature change occurs when. Endothermic Reaction Temperature Decrease.
From general.chemistrysteps.com
The Effect of 𝚫H, 𝚫S, and T on 𝚫G Spontaneity Chemistry Steps Endothermic Reaction Temperature Decrease When energy is released in an. in an endothermic change, temperature is absorbed from surrounding molecules to continue reacting. a temperature change occurs when temperature is increased or decreased by the flow of heat. how can decreasing in temperature indicate an endothermic reaction? an endothermic reaction occurs when the temperature of an isolated system decreases while. Endothermic Reaction Temperature Decrease.
From www.slideserve.com
PPT Topic 5 Chemical Reactions Revision Checklist PowerPoint Endothermic Reaction Temperature Decrease (6 answers) closed 3 years ago. a thermometer is used to detect the temperature decrease. in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings. in an endothermic change, temperature is absorbed from surrounding molecules to continue reacting. an endothermic reaction occurs when the temperature. Endothermic Reaction Temperature Decrease.
From www.yaclass.in
Exothermic and Endothermic chemical changes — lesson. Science State Endothermic Reaction Temperature Decrease an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. a temperature change occurs when temperature is increased or decreased by the flow of heat. a thermometer is used to detect the temperature decrease. how can decreasing in temperature indicate an endothermic reaction? (6 answers) closed 3 years ago. in. Endothermic Reaction Temperature Decrease.
From lessonschoolallodial.z13.web.core.windows.net
Endothermic Vs Exothermic Chemical Reactions Endothermic Reaction Temperature Decrease (6 answers) closed 3 years ago. in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings. Some examples of endothermic reactions are: When energy is released in an. a thermometer is used to detect the temperature decrease. in an endothermic change, temperature is absorbed from surrounding. Endothermic Reaction Temperature Decrease.
From www.slideserve.com
PPT Endothermic Vs. Exothermic Reaction Graphs PowerPoint Endothermic Reaction Temperature Decrease an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. a thermometer is used to detect the temperature decrease. When energy is released in an. there are two methods for distinguishing between exothermic and endothermic reactions. Some examples of endothermic reactions are: how can decreasing in temperature indicate an endothermic reaction?. Endothermic Reaction Temperature Decrease.
From general.chemistrysteps.com
Entropy Changes in the Surroundings Chemistry Steps Endothermic Reaction Temperature Decrease in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings. (6 answers) closed 3 years ago. Some examples of endothermic reactions are: in an endothermic change, temperature is absorbed from surrounding molecules to continue reacting. a thermometer is used to detect the temperature decrease. a. Endothermic Reaction Temperature Decrease.
From www.slideserve.com
PPT Le Chatelier’s Principle PowerPoint Presentation, free download Endothermic Reaction Temperature Decrease in an endothermic change, temperature is absorbed from surrounding molecules to continue reacting. in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings. how can decreasing in temperature indicate an endothermic reaction? a temperature change occurs when temperature is increased or decreased by the flow. Endothermic Reaction Temperature Decrease.
From mmerevise.co.uk
Enthalpy Changes and Calorimetry MME Endothermic Reaction Temperature Decrease When energy is released in an. there are two methods for distinguishing between exothermic and endothermic reactions. an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings. Some examples of endothermic. Endothermic Reaction Temperature Decrease.
From musicbykatie.com
Does Temperature Increase Or Decrease Solubility In Endothermic Endothermic Reaction Temperature Decrease a thermometer is used to detect the temperature decrease. there are two methods for distinguishing between exothermic and endothermic reactions. When energy is released in an. a temperature change occurs when temperature is increased or decreased by the flow of heat. how can decreasing in temperature indicate an endothermic reaction? in the course of an. Endothermic Reaction Temperature Decrease.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Endothermic Reaction Temperature Decrease in an endothermic change, temperature is absorbed from surrounding molecules to continue reacting. an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. a thermometer is used to detect the temperature decrease. in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of. Endothermic Reaction Temperature Decrease.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction Temperature Decrease an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. how can decreasing in temperature indicate an endothermic reaction? there are two methods for distinguishing between exothermic and endothermic reactions. in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings.. Endothermic Reaction Temperature Decrease.
From evulpo.com
Endothermic and exothermic reactions Chemistry Explanation Endothermic Reaction Temperature Decrease in an endothermic change, temperature is absorbed from surrounding molecules to continue reacting. When energy is released in an. Some examples of endothermic reactions are: there are two methods for distinguishing between exothermic and endothermic reactions. an endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings. (6 answers) closed 3 years ago.. Endothermic Reaction Temperature Decrease.