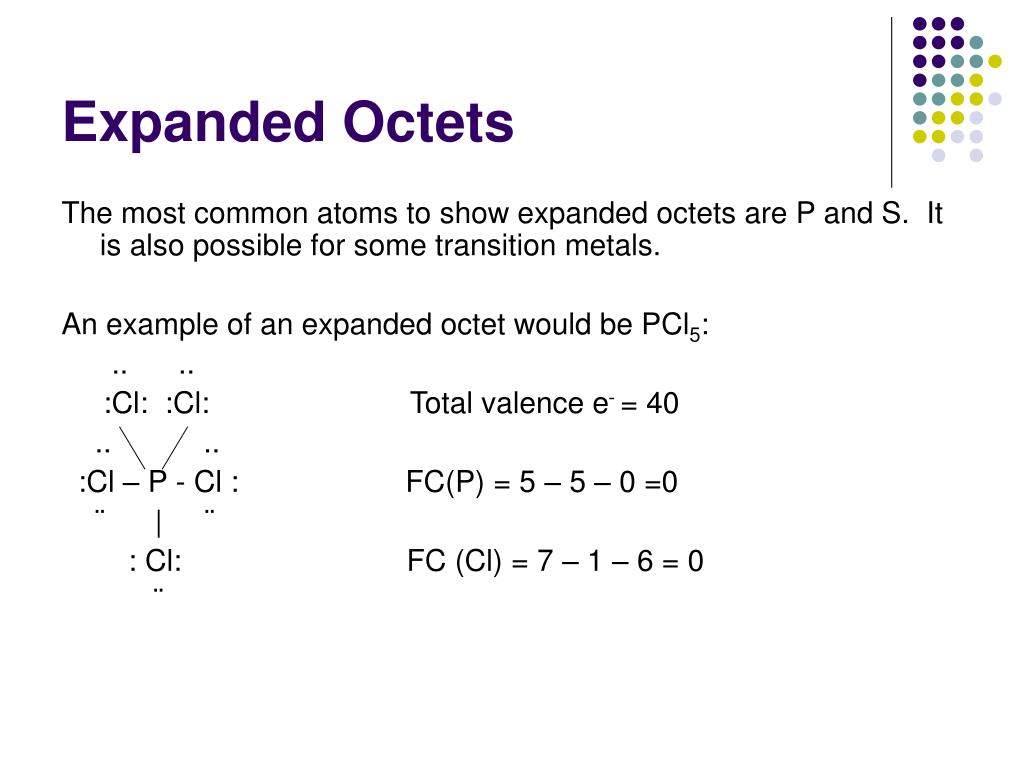
Why Do Some Atoms Have Expanded Octets . When atoms contain more than eight electrons in their valence shell, they are said to be hypervalent. Find out which elements follow this rule and how it affects their. Learn about the expanded octet rule, which allows some atoms to have more than eight electrons in their valence shells. Learn what an expanded octet is and how it occurs in molecules or ions with elements from the third period and beyond. The total number of valence electrons is. Find out how atoms can have reduced or expanded octets, and why some elements cannot have an expanded octet. The octet rule is due to the eight electrons allowed in the s. The octet of the central sulfur atom has been expanded to hold 10 electrons. Learn about the octet rule, which says that many compounds have 8 electrons in their valence shells, and its exceptions. Learn about the three categories of exceptions to the octet rule in covalent bonding:
from www.slideserve.com
Learn about the expanded octet rule, which allows some atoms to have more than eight electrons in their valence shells. The octet rule is due to the eight electrons allowed in the s. Find out which elements follow this rule and how it affects their. When atoms contain more than eight electrons in their valence shell, they are said to be hypervalent. Learn about the three categories of exceptions to the octet rule in covalent bonding: Find out how atoms can have reduced or expanded octets, and why some elements cannot have an expanded octet. Learn what an expanded octet is and how it occurs in molecules or ions with elements from the third period and beyond. Learn about the octet rule, which says that many compounds have 8 electrons in their valence shells, and its exceptions. The total number of valence electrons is. The octet of the central sulfur atom has been expanded to hold 10 electrons.
PPT Lewis Dot Structures PowerPoint Presentation, free download ID
Why Do Some Atoms Have Expanded Octets Learn about the three categories of exceptions to the octet rule in covalent bonding: The total number of valence electrons is. The octet rule is due to the eight electrons allowed in the s. Find out which elements follow this rule and how it affects their. When atoms contain more than eight electrons in their valence shell, they are said to be hypervalent. The octet of the central sulfur atom has been expanded to hold 10 electrons. Learn what an expanded octet is and how it occurs in molecules or ions with elements from the third period and beyond. Find out how atoms can have reduced or expanded octets, and why some elements cannot have an expanded octet. Learn about the octet rule, which says that many compounds have 8 electrons in their valence shells, and its exceptions. Learn about the three categories of exceptions to the octet rule in covalent bonding: Learn about the expanded octet rule, which allows some atoms to have more than eight electrons in their valence shells.
From www.animalia-life.club
Expanded Octet Why Do Some Atoms Have Expanded Octets Find out which elements follow this rule and how it affects their. Find out how atoms can have reduced or expanded octets, and why some elements cannot have an expanded octet. Learn about the three categories of exceptions to the octet rule in covalent bonding: The octet of the central sulfur atom has been expanded to hold 10 electrons. Learn. Why Do Some Atoms Have Expanded Octets.
From www.youtube.com
Expanded Octet Lewis Structures YouTube Why Do Some Atoms Have Expanded Octets Learn about the expanded octet rule, which allows some atoms to have more than eight electrons in their valence shells. The octet rule is due to the eight electrons allowed in the s. Learn what an expanded octet is and how it occurs in molecules or ions with elements from the third period and beyond. The octet of the central. Why Do Some Atoms Have Expanded Octets.
From www.slideserve.com
PPT 1 10 11 12 15 17 20 24 31 37 39 49 53 55 61 65 67 71 88 103 Why Do Some Atoms Have Expanded Octets Learn about the octet rule, which says that many compounds have 8 electrons in their valence shells, and its exceptions. The octet rule is due to the eight electrons allowed in the s. Learn about the expanded octet rule, which allows some atoms to have more than eight electrons in their valence shells. Learn what an expanded octet is and. Why Do Some Atoms Have Expanded Octets.
From www.slideserve.com
PPT Today’s Class PowerPoint Presentation, free download ID463502 Why Do Some Atoms Have Expanded Octets The total number of valence electrons is. Learn about the expanded octet rule, which allows some atoms to have more than eight electrons in their valence shells. Learn about the three categories of exceptions to the octet rule in covalent bonding: When atoms contain more than eight electrons in their valence shell, they are said to be hypervalent. The octet. Why Do Some Atoms Have Expanded Octets.
From studylib.net
Expanded valence shells (extended octets) Why Do Some Atoms Have Expanded Octets The total number of valence electrons is. Learn about the expanded octet rule, which allows some atoms to have more than eight electrons in their valence shells. The octet rule is due to the eight electrons allowed in the s. Learn about the octet rule, which says that many compounds have 8 electrons in their valence shells, and its exceptions.. Why Do Some Atoms Have Expanded Octets.
From joaquin-ktyler.blogspot.com
Describe How the Octet Rule Applies to Covalent Bonds. Why Do Some Atoms Have Expanded Octets Find out which elements follow this rule and how it affects their. The octet rule is due to the eight electrons allowed in the s. The octet of the central sulfur atom has been expanded to hold 10 electrons. Learn what an expanded octet is and how it occurs in molecules or ions with elements from the third period and. Why Do Some Atoms Have Expanded Octets.
From www.animalia-life.club
Expanded Octet Why Do Some Atoms Have Expanded Octets The octet rule is due to the eight electrons allowed in the s. When atoms contain more than eight electrons in their valence shell, they are said to be hypervalent. Learn about the octet rule, which says that many compounds have 8 electrons in their valence shells, and its exceptions. The total number of valence electrons is. Learn about the. Why Do Some Atoms Have Expanded Octets.
From ar.inspiredpencil.com
Expanded Octet Periodic Table Why Do Some Atoms Have Expanded Octets Learn what an expanded octet is and how it occurs in molecules or ions with elements from the third period and beyond. Learn about the expanded octet rule, which allows some atoms to have more than eight electrons in their valence shells. The total number of valence electrons is. The octet rule is due to the eight electrons allowed in. Why Do Some Atoms Have Expanded Octets.
From www.numerade.com
SOLVEDEach molecule listed contains an expanded octet (10 or 12 Why Do Some Atoms Have Expanded Octets Learn about the octet rule, which says that many compounds have 8 electrons in their valence shells, and its exceptions. Find out which elements follow this rule and how it affects their. Find out how atoms can have reduced or expanded octets, and why some elements cannot have an expanded octet. Learn about the three categories of exceptions to the. Why Do Some Atoms Have Expanded Octets.
From www.slideserve.com
PPT Ch 9 Bonding and Molecular Structure Fundamental Concepts Why Do Some Atoms Have Expanded Octets Learn about the expanded octet rule, which allows some atoms to have more than eight electrons in their valence shells. When atoms contain more than eight electrons in their valence shell, they are said to be hypervalent. Learn what an expanded octet is and how it occurs in molecules or ions with elements from the third period and beyond. Learn. Why Do Some Atoms Have Expanded Octets.
From studymultiloquy.z21.web.core.windows.net
Expanded Octet Explained Why Do Some Atoms Have Expanded Octets The octet of the central sulfur atom has been expanded to hold 10 electrons. Learn about the octet rule, which says that many compounds have 8 electrons in their valence shells, and its exceptions. Learn about the expanded octet rule, which allows some atoms to have more than eight electrons in their valence shells. Find out how atoms can have. Why Do Some Atoms Have Expanded Octets.
From www.vrogue.co
Illustrated Glossary Of Organic Chemistry Expanded Oc vrogue.co Why Do Some Atoms Have Expanded Octets The octet of the central sulfur atom has been expanded to hold 10 electrons. When atoms contain more than eight electrons in their valence shell, they are said to be hypervalent. Learn about the three categories of exceptions to the octet rule in covalent bonding: Learn what an expanded octet is and how it occurs in molecules or ions with. Why Do Some Atoms Have Expanded Octets.
From educationgrafts.z21.web.core.windows.net
What Is An Expanded Octet Why Do Some Atoms Have Expanded Octets The total number of valence electrons is. Learn about the three categories of exceptions to the octet rule in covalent bonding: When atoms contain more than eight electrons in their valence shell, they are said to be hypervalent. Find out how atoms can have reduced or expanded octets, and why some elements cannot have an expanded octet. Find out which. Why Do Some Atoms Have Expanded Octets.
From www.youtube.com
AP Chemistry Lewis Structures and Formal Charges and expanded octets Why Do Some Atoms Have Expanded Octets The octet of the central sulfur atom has been expanded to hold 10 electrons. Learn about the octet rule, which says that many compounds have 8 electrons in their valence shells, and its exceptions. When atoms contain more than eight electrons in their valence shell, they are said to be hypervalent. Learn what an expanded octet is and how it. Why Do Some Atoms Have Expanded Octets.
From www.slideserve.com
PPT Molecular structure and naming PowerPoint Presentation, free Why Do Some Atoms Have Expanded Octets Learn what an expanded octet is and how it occurs in molecules or ions with elements from the third period and beyond. Find out how atoms can have reduced or expanded octets, and why some elements cannot have an expanded octet. When atoms contain more than eight electrons in their valence shell, they are said to be hypervalent. Find out. Why Do Some Atoms Have Expanded Octets.
From slideplayer.com
Chapter 7 Ionic & Covalent Bonds. ppt download Why Do Some Atoms Have Expanded Octets Learn about the expanded octet rule, which allows some atoms to have more than eight electrons in their valence shells. The octet rule is due to the eight electrons allowed in the s. When atoms contain more than eight electrons in their valence shell, they are said to be hypervalent. Learn what an expanded octet is and how it occurs. Why Do Some Atoms Have Expanded Octets.
From www.youtube.com
Lesson 6.1cLewis Dot Structures Expanded Octets & Resonance YouTube Why Do Some Atoms Have Expanded Octets The octet rule is due to the eight electrons allowed in the s. When atoms contain more than eight electrons in their valence shell, they are said to be hypervalent. Learn about the octet rule, which says that many compounds have 8 electrons in their valence shells, and its exceptions. Learn what an expanded octet is and how it occurs. Why Do Some Atoms Have Expanded Octets.
From www.slideserve.com
PPT Lewis Dot Structures PowerPoint Presentation, free download ID Why Do Some Atoms Have Expanded Octets The total number of valence electrons is. Find out how atoms can have reduced or expanded octets, and why some elements cannot have an expanded octet. When atoms contain more than eight electrons in their valence shell, they are said to be hypervalent. The octet rule is due to the eight electrons allowed in the s. The octet of the. Why Do Some Atoms Have Expanded Octets.
From www.slideserve.com
PPT Ch 9 Bonding and Molecular Structure Fundamental Concepts Why Do Some Atoms Have Expanded Octets Find out how atoms can have reduced or expanded octets, and why some elements cannot have an expanded octet. The total number of valence electrons is. Learn about the three categories of exceptions to the octet rule in covalent bonding: The octet rule is due to the eight electrons allowed in the s. Learn about the octet rule, which says. Why Do Some Atoms Have Expanded Octets.
From www.rucete.me
The Electron Configuration of Atoms Lewis Dot diagrams, Why Do Some Atoms Have Expanded Octets When atoms contain more than eight electrons in their valence shell, they are said to be hypervalent. The octet rule is due to the eight electrons allowed in the s. Learn about the octet rule, which says that many compounds have 8 electrons in their valence shells, and its exceptions. Find out which elements follow this rule and how it. Why Do Some Atoms Have Expanded Octets.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Octet rule Why Do Some Atoms Have Expanded Octets When atoms contain more than eight electrons in their valence shell, they are said to be hypervalent. The total number of valence electrons is. Learn about the octet rule, which says that many compounds have 8 electrons in their valence shells, and its exceptions. Find out which elements follow this rule and how it affects their. Learn about the three. Why Do Some Atoms Have Expanded Octets.
From www.coursehero.com
The Expanded Octet Introduction to Chemistry Course Hero Why Do Some Atoms Have Expanded Octets Learn about the three categories of exceptions to the octet rule in covalent bonding: Learn about the octet rule, which says that many compounds have 8 electrons in their valence shells, and its exceptions. The octet of the central sulfur atom has been expanded to hold 10 electrons. When atoms contain more than eight electrons in their valence shell, they. Why Do Some Atoms Have Expanded Octets.
From www.animalia-life.club
Expanded Octet Why Do Some Atoms Have Expanded Octets The total number of valence electrons is. Find out how atoms can have reduced or expanded octets, and why some elements cannot have an expanded octet. Learn about the three categories of exceptions to the octet rule in covalent bonding: The octet rule is due to the eight electrons allowed in the s. Learn about the octet rule, which says. Why Do Some Atoms Have Expanded Octets.
From www.slideshare.net
Me cchapter 3 Why Do Some Atoms Have Expanded Octets Find out which elements follow this rule and how it affects their. The octet rule is due to the eight electrons allowed in the s. Find out how atoms can have reduced or expanded octets, and why some elements cannot have an expanded octet. Learn about the three categories of exceptions to the octet rule in covalent bonding: Learn what. Why Do Some Atoms Have Expanded Octets.
From sciencenotes.org
Octet Rule Definition, Examples, and Exceptions Why Do Some Atoms Have Expanded Octets The octet of the central sulfur atom has been expanded to hold 10 electrons. Learn about the octet rule, which says that many compounds have 8 electrons in their valence shells, and its exceptions. The total number of valence electrons is. Learn about the expanded octet rule, which allows some atoms to have more than eight electrons in their valence. Why Do Some Atoms Have Expanded Octets.
From quizquadratrix.z21.web.core.windows.net
What Is An Expanded Octet Why Do Some Atoms Have Expanded Octets Learn about the expanded octet rule, which allows some atoms to have more than eight electrons in their valence shells. Learn what an expanded octet is and how it occurs in molecules or ions with elements from the third period and beyond. The octet of the central sulfur atom has been expanded to hold 10 electrons. When atoms contain more. Why Do Some Atoms Have Expanded Octets.
From rumble.com
Lewis diagrams, expanded octet Chemistry Why Do Some Atoms Have Expanded Octets Learn about the three categories of exceptions to the octet rule in covalent bonding: When atoms contain more than eight electrons in their valence shell, they are said to be hypervalent. Learn about the octet rule, which says that many compounds have 8 electrons in their valence shells, and its exceptions. Learn what an expanded octet is and how it. Why Do Some Atoms Have Expanded Octets.
From www.coursehero.com
The Expanded Octet Introduction to Chemistry Course Hero Why Do Some Atoms Have Expanded Octets When atoms contain more than eight electrons in their valence shell, they are said to be hypervalent. Find out how atoms can have reduced or expanded octets, and why some elements cannot have an expanded octet. Learn about the three categories of exceptions to the octet rule in covalent bonding: Learn what an expanded octet is and how it occurs. Why Do Some Atoms Have Expanded Octets.
From www.studocu.com
Expanded Octets with Cl CHEM12A Studocu Why Do Some Atoms Have Expanded Octets The octet rule is due to the eight electrons allowed in the s. Learn about the three categories of exceptions to the octet rule in covalent bonding: When atoms contain more than eight electrons in their valence shell, they are said to be hypervalent. The octet of the central sulfur atom has been expanded to hold 10 electrons. Learn about. Why Do Some Atoms Have Expanded Octets.
From www.youtube.com
Lewis Structures III Expanded octets YouTube Why Do Some Atoms Have Expanded Octets The octet rule is due to the eight electrons allowed in the s. Learn about the expanded octet rule, which allows some atoms to have more than eight electrons in their valence shells. Learn about the three categories of exceptions to the octet rule in covalent bonding: Learn about the octet rule, which says that many compounds have 8 electrons. Why Do Some Atoms Have Expanded Octets.
From www.showme.com
8.9a Expanded Octets Science, Chemistry ShowMe Why Do Some Atoms Have Expanded Octets Find out how atoms can have reduced or expanded octets, and why some elements cannot have an expanded octet. Find out which elements follow this rule and how it affects their. Learn about the three categories of exceptions to the octet rule in covalent bonding: The total number of valence electrons is. Learn about the octet rule, which says that. Why Do Some Atoms Have Expanded Octets.
From www.youtube.com
Lewis Structures Part 3 Expanded Octets YouTube Why Do Some Atoms Have Expanded Octets Find out how atoms can have reduced or expanded octets, and why some elements cannot have an expanded octet. The octet of the central sulfur atom has been expanded to hold 10 electrons. Learn about the octet rule, which says that many compounds have 8 electrons in their valence shells, and its exceptions. Learn what an expanded octet is and. Why Do Some Atoms Have Expanded Octets.
From animalia-life.club
Expanded Octet Why Do Some Atoms Have Expanded Octets The octet rule is due to the eight electrons allowed in the s. Learn about the three categories of exceptions to the octet rule in covalent bonding: When atoms contain more than eight electrons in their valence shell, they are said to be hypervalent. The total number of valence electrons is. Find out which elements follow this rule and how. Why Do Some Atoms Have Expanded Octets.
From www.slideserve.com
PPT More Lewis Structures PowerPoint Presentation, free download ID Why Do Some Atoms Have Expanded Octets Find out which elements follow this rule and how it affects their. Learn about the three categories of exceptions to the octet rule in covalent bonding: The octet rule is due to the eight electrons allowed in the s. Learn about the expanded octet rule, which allows some atoms to have more than eight electrons in their valence shells. Learn. Why Do Some Atoms Have Expanded Octets.
From www.slideserve.com
PPT Intersection 4 PowerPoint Presentation, free download ID6313576 Why Do Some Atoms Have Expanded Octets The octet rule is due to the eight electrons allowed in the s. Learn about the expanded octet rule, which allows some atoms to have more than eight electrons in their valence shells. Learn what an expanded octet is and how it occurs in molecules or ions with elements from the third period and beyond. Learn about the octet rule,. Why Do Some Atoms Have Expanded Octets.