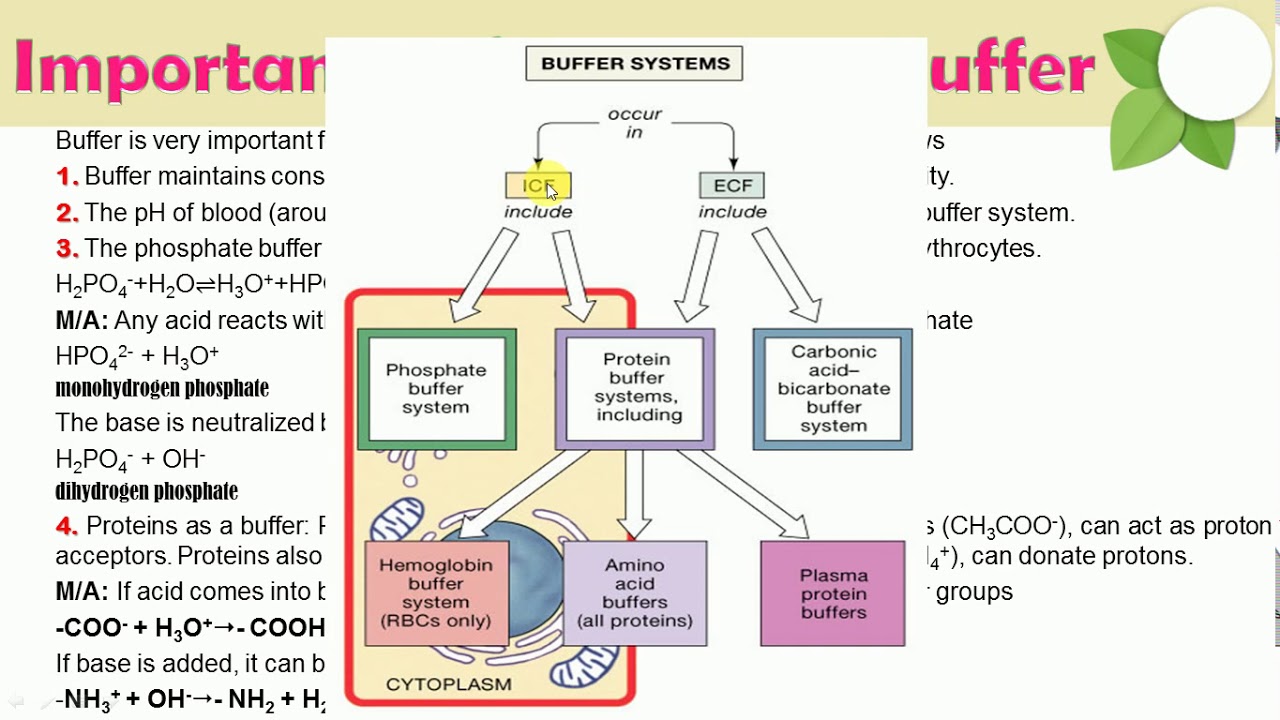
Buffer Importance In Biological Systems . the use of buffers that mimic biological solutions is a foundation of biochemical studies. you should be aware that buffers play a critical role in almost all biochemical systems. One of the most common. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. hence, we highlight the importance of buffers in studies related to ion specific ‘hofmeister effects’ in.
from www.youtube.com
the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. the use of buffers that mimic biological solutions is a foundation of biochemical studies. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. you should be aware that buffers play a critical role in almost all biochemical systems. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. One of the most common. hence, we highlight the importance of buffers in studies related to ion specific ‘hofmeister effects’ in.
Buffer Capacity 03 Buffers in pharmaceutical and biological systems
Buffer Importance In Biological Systems the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the use of buffers that mimic biological solutions is a foundation of biochemical studies. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. you should be aware that buffers play a critical role in almost all biochemical systems. hence, we highlight the importance of buffers in studies related to ion specific ‘hofmeister effects’ in. One of the most common.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Buffer Importance In Biological Systems the use of buffers that mimic biological solutions is a foundation of biochemical studies. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. hence, we highlight the importance of buffers in studies. Buffer Importance In Biological Systems.
From www.youtube.com
Role of buffers in biological system YouTube Buffer Importance In Biological Systems hence, we highlight the importance of buffers in studies related to ion specific ‘hofmeister effects’ in. the use of buffers that mimic biological solutions is a foundation of biochemical studies. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the purpose of a buffer in a biological system. Buffer Importance In Biological Systems.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID Buffer Importance In Biological Systems One of the most common. hence, we highlight the importance of buffers in studies related to ion specific ‘hofmeister effects’ in. the use of buffers that mimic biological solutions is a foundation of biochemical studies. you should be aware that buffers play a critical role in almost all biochemical systems. the buffer systems functioning in blood. Buffer Importance In Biological Systems.
From questions.kunduz.com
Two important biological buffer systems c... Physical Chemistry Buffer Importance In Biological Systems the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. hence, we highlight the importance of buffers in studies related to ion specific ‘hofmeister effects’ in. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. the most relevant. Buffer Importance In Biological Systems.
From fyoqfobay.blob.core.windows.net
Biological Buffer System Examples at Michele Huff blog Buffer Importance In Biological Systems the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. you should be aware that buffers play a critical role in almost all biochemical systems. One of the most common. hence, we highlight the importance of buffers in studies related to ion specific ‘hofmeister effects’ in. the use of. Buffer Importance In Biological Systems.
From www.pinterest.com
What are the Buffers and its Importance? This article explains the Buffer Importance In Biological Systems hence, we highlight the importance of buffers in studies related to ion specific ‘hofmeister effects’ in. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. you should be. Buffer Importance In Biological Systems.
From www.coursehero.com
[Solved] Slide 6 7 Davi 22. Why are buffers important in biological Buffer Importance In Biological Systems you should be aware that buffers play a critical role in almost all biochemical systems. hence, we highlight the importance of buffers in studies related to ion specific ‘hofmeister effects’ in. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. the use of buffers that. Buffer Importance In Biological Systems.
From fyoqfobay.blob.core.windows.net
Biological Buffer System Examples at Michele Huff blog Buffer Importance In Biological Systems the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. One of the most common. you should be aware that buffers play a critical role in almost all biochemical systems. hence, we highlight the importance of buffers in studies related to ion specific ‘hofmeister effects’ in. the purpose of a. Buffer Importance In Biological Systems.
From www.slideserve.com
PPT Acids and Bases and Buffers PowerPoint Presentation, free Buffer Importance In Biological Systems the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. the use of buffers that mimic biological solutions is a foundation of biochemical studies. you should be aware that buffers play a critical. Buffer Importance In Biological Systems.
From www.studocu.com
Biological Buffer Systems BIOLOGICAL BUFFER SYSTEMS Almost every Buffer Importance In Biological Systems the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. the use of buffers that mimic biological solutions is a foundation of biochemical studies. you should be aware that buffers play a critical role in almost all biochemical systems. the buffer systems functioning in blood plasma include plasma proteins, phosphate,. Buffer Importance In Biological Systems.
From www.slideserve.com
PPT Role of Buffers PowerPoint Presentation, free download ID1883450 Buffer Importance In Biological Systems the use of buffers that mimic biological solutions is a foundation of biochemical studies. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. One of the most common. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. . Buffer Importance In Biological Systems.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Buffer Importance In Biological Systems the use of buffers that mimic biological solutions is a foundation of biochemical studies. One of the most common. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. you should be aware that buffers play a critical role in almost all biochemical systems. the most relevant systems for. Buffer Importance In Biological Systems.
From www.slideserve.com
PPT UNIT IV PowerPoint Presentation, free download ID4510549 Buffer Importance In Biological Systems the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. hence, we highlight the importance of buffers in studies related to ion specific ‘hofmeister effects’ in. One of the most common. the use of buffers that mimic biological solutions is a foundation of biochemical studies. the most relevant systems. Buffer Importance In Biological Systems.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Buffer Importance In Biological Systems the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. you should be aware that buffers play a critical role in almost all biochemical systems. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. the most relevant systems. Buffer Importance In Biological Systems.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Buffer Importance In Biological Systems hence, we highlight the importance of buffers in studies related to ion specific ‘hofmeister effects’ in. you should be aware that buffers play a critical role in almost all biochemical systems. the use of buffers that mimic biological solutions is a foundation of biochemical studies. the buffer systems functioning in blood plasma include plasma proteins, phosphate,. Buffer Importance In Biological Systems.
From www.slideserve.com
PPT UNIT IV PowerPoint Presentation, free download ID4510549 Buffer Importance In Biological Systems you should be aware that buffers play a critical role in almost all biochemical systems. the use of buffers that mimic biological solutions is a foundation of biochemical studies. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. the buffer systems functioning in blood plasma. Buffer Importance In Biological Systems.
From www.youtube.com
Introduction to Buffer System Regulation of pH Acid Base Balance Buffer Importance In Biological Systems One of the most common. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. you should be aware that buffers play a critical role in almost all biochemical systems. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers.. Buffer Importance In Biological Systems.
From www.slideserve.com
PPT UNIT IV PowerPoint Presentation, free download ID4510549 Buffer Importance In Biological Systems the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. you should be aware that buffers play a critical role in almost all biochemical systems. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. hence, we highlight the. Buffer Importance In Biological Systems.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Buffer Importance In Biological Systems the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. you should be aware that buffers play a critical role in almost all biochemical systems. One of the most common.. Buffer Importance In Biological Systems.
From www.youtube.com
buffer isotonic solution buffer capacity buffers in pharmaceutical Buffer Importance In Biological Systems hence, we highlight the importance of buffers in studies related to ion specific ‘hofmeister effects’ in. the use of buffers that mimic biological solutions is a foundation of biochemical studies. you should be aware that buffers play a critical role in almost all biochemical systems. the purpose of a buffer in a biological system is to. Buffer Importance In Biological Systems.
From www.slideshare.net
Buffers in biological systems Buffer Importance In Biological Systems the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. the use of buffers that mimic biological solutions is a foundation of biochemical studies. you should be aware that buffers play a critical role in almost all biochemical systems. the buffer systems functioning in blood plasma include plasma proteins, phosphate,. Buffer Importance In Biological Systems.
From www.slideserve.com
PPT 8.3 Bases PowerPoint Presentation, free download ID5917783 Buffer Importance In Biological Systems the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. the use of buffers that mimic biological solutions is a foundation of biochemical studies. hence, we highlight the importance of buffers in studies related to ion specific ‘hofmeister effects’ in. One of the most common. the buffer systems functioning in. Buffer Importance In Biological Systems.
From www.studypool.com
SOLUTION Biological buffer systems Studypool Buffer Importance In Biological Systems the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. hence, we highlight the importance of buffers in studies related to ion specific ‘hofmeister effects’ in. you should be aware that buffers play a critical role in almost all biochemical systems. the purpose of a buffer in a biological. Buffer Importance In Biological Systems.
From www.slideshare.net
Buffers in biological systems Buffer Importance In Biological Systems the use of buffers that mimic biological solutions is a foundation of biochemical studies. you should be aware that buffers play a critical role in almost all biochemical systems. hence, we highlight the importance of buffers in studies related to ion specific ‘hofmeister effects’ in. One of the most common. the buffer systems functioning in blood. Buffer Importance In Biological Systems.
From fyopwxzrh.blob.core.windows.net
Biological Buffer Define at Jennifer Barraza blog Buffer Importance In Biological Systems hence, we highlight the importance of buffers in studies related to ion specific ‘hofmeister effects’ in. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. the use of buffers that mimic biological. Buffer Importance In Biological Systems.
From www.slideserve.com
PPT Physiological system of blood. Functional importance of blood Buffer Importance In Biological Systems One of the most common. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. you should be aware that buffers play a critical role in almost all biochemical systems. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers.. Buffer Importance In Biological Systems.
From www.leinco.com
Biological Buffers And pH Range Leinco Technologies Buffer Importance In Biological Systems the use of buffers that mimic biological solutions is a foundation of biochemical studies. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. One of the most common. . Buffer Importance In Biological Systems.
From www.slideserve.com
PPT Blood Gases, pH and Buffer system PowerPoint Presentation ID257615 Buffer Importance In Biological Systems the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. the use of buffers that mimic biological solutions is a foundation of biochemical studies. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. hence, we highlight the importance of. Buffer Importance In Biological Systems.
From www.chegg.com
Solved 13. Buffers are common (and extremely important) in Buffer Importance In Biological Systems hence, we highlight the importance of buffers in studies related to ion specific ‘hofmeister effects’ in. the use of buffers that mimic biological solutions is a foundation of biochemical studies. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. One of the most common. you should be aware. Buffer Importance In Biological Systems.
From www.chegg.com
Solved Explain why buffers are important in biological Buffer Importance In Biological Systems the use of buffers that mimic biological solutions is a foundation of biochemical studies. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. One of the most common. . Buffer Importance In Biological Systems.
From www.slideserve.com
PPT Buffers in Blood. Acidosis and Alkalosis. PowerPoint Presentation Buffer Importance In Biological Systems hence, we highlight the importance of buffers in studies related to ion specific ‘hofmeister effects’ in. you should be aware that buffers play a critical role in almost all biochemical systems. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. the use of buffers that. Buffer Importance In Biological Systems.
From www.youtube.com
Buffer Capacity 03 Buffers in pharmaceutical and biological systems Buffer Importance In Biological Systems the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. the use of buffers that mimic biological solutions is a foundation of biochemical studies. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. you should be aware that. Buffer Importance In Biological Systems.
From slidetodoc.com
Regulation of Extracellular Fluid Osmolarity and Sodium Concentration Buffer Importance In Biological Systems the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. you should be aware that buffers play a critical role in almost all biochemical systems. One of the most common. . Buffer Importance In Biological Systems.
From www.youtube.com
Buffer in biological system buffer system in blood buffer in eyes Buffer Importance In Biological Systems the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. One of the most common. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very. Buffer Importance In Biological Systems.
From www.slideshare.net
Buffer system Buffer Importance In Biological Systems you should be aware that buffers play a critical role in almost all biochemical systems. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. One of the most common. hence, we highlight the importance of buffers in studies related to ion specific ‘hofmeister effects’ in. the use of buffers. Buffer Importance In Biological Systems.