Magnesium Carbonate In Water . Magnesium carbonate is an inorganic salt with the chemical formula mgco3. All forms of magnesium carbonate dissolve in acids. Magnesium carbonate is used as an antacid which reduces stomach acid. Also small amounts of magnesium carbonate (about 6% of the magnesium supplied by rivers) are deposited with calcite (caco3) in seawater. When it comes to identifying magnesium carbonate, water solubility is just one of many properties that identify it. Students react magnesium sulfate and sodium carbonate to form magnesium carbonate, which is insoluble in water. Magnesium carbonate is generally recognized as a white, solid compound that is insoluble in water but readily dissolves in acids. Includes kit list and safety instructions. Magnesium carbonate, for example, has a solubility of about 0.02 g per 100 g of water at room temperature. There is little data for. The anhydrous salt is practically insoluble in water, acetone, and ammonia. Usually, this substance is positively identified through its chemical. There is significant uptake of.
from www.numerade.com
When it comes to identifying magnesium carbonate, water solubility is just one of many properties that identify it. Magnesium carbonate is used as an antacid which reduces stomach acid. There is little data for. There is significant uptake of. Magnesium carbonate, for example, has a solubility of about 0.02 g per 100 g of water at room temperature. Usually, this substance is positively identified through its chemical. Students react magnesium sulfate and sodium carbonate to form magnesium carbonate, which is insoluble in water. Includes kit list and safety instructions. All forms of magnesium carbonate dissolve in acids. Also small amounts of magnesium carbonate (about 6% of the magnesium supplied by rivers) are deposited with calcite (caco3) in seawater.
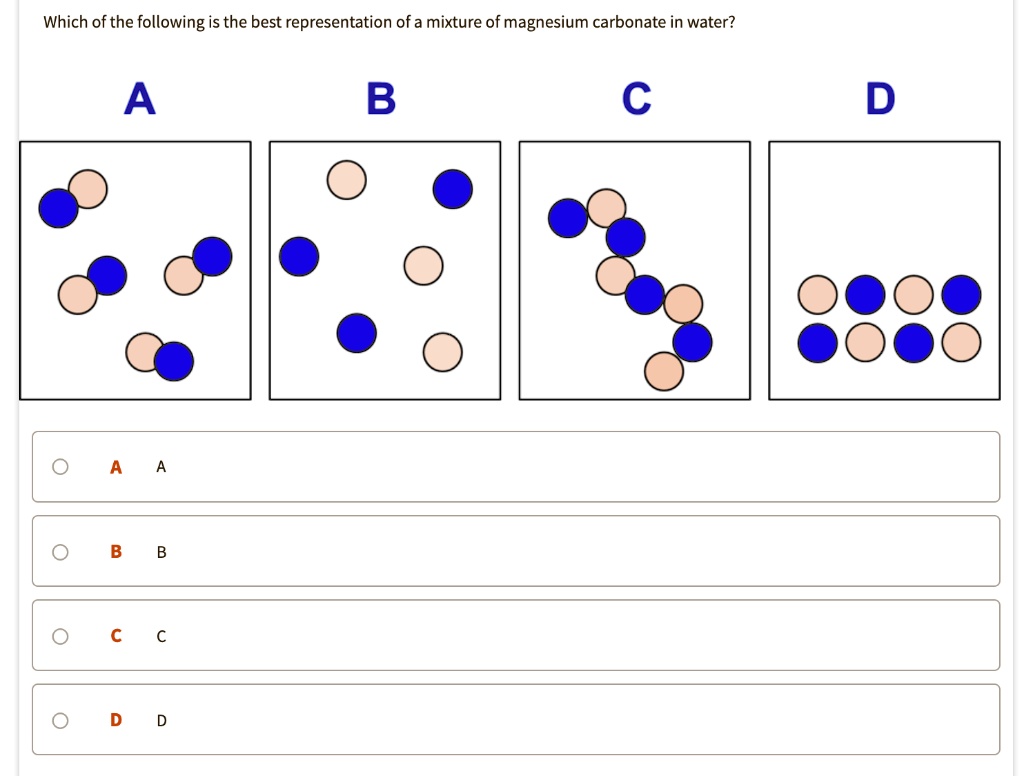
SOLVED Which ofthe following is the best representation of a mixture
Magnesium Carbonate In Water Usually, this substance is positively identified through its chemical. Magnesium carbonate, for example, has a solubility of about 0.02 g per 100 g of water at room temperature. Magnesium carbonate is used as an antacid which reduces stomach acid. The anhydrous salt is practically insoluble in water, acetone, and ammonia. There is little data for. Also small amounts of magnesium carbonate (about 6% of the magnesium supplied by rivers) are deposited with calcite (caco3) in seawater. When it comes to identifying magnesium carbonate, water solubility is just one of many properties that identify it. Magnesium carbonate is generally recognized as a white, solid compound that is insoluble in water but readily dissolves in acids. Students react magnesium sulfate and sodium carbonate to form magnesium carbonate, which is insoluble in water. There is significant uptake of. All forms of magnesium carbonate dissolve in acids. Magnesium carbonate is an inorganic salt with the chemical formula mgco3. Usually, this substance is positively identified through its chemical. Includes kit list and safety instructions.
From www.degruyter.com
Synthesis of magnesium carbonate hydrate from natural talc Magnesium Carbonate In Water Usually, this substance is positively identified through its chemical. When it comes to identifying magnesium carbonate, water solubility is just one of many properties that identify it. The anhydrous salt is practically insoluble in water, acetone, and ammonia. Magnesium carbonate, for example, has a solubility of about 0.02 g per 100 g of water at room temperature. Includes kit list. Magnesium Carbonate In Water.
From janicerwesto.blob.core.windows.net
Magnesium Bicarbonate Function at janicerwesto blog Magnesium Carbonate In Water There is little data for. Includes kit list and safety instructions. All forms of magnesium carbonate dissolve in acids. Magnesium carbonate is an inorganic salt with the chemical formula mgco3. Magnesium carbonate, for example, has a solubility of about 0.02 g per 100 g of water at room temperature. The anhydrous salt is practically insoluble in water, acetone, and ammonia.. Magnesium Carbonate In Water.
From yesdirt.com
Is Magnesium Carbonate Soluble in Water? (Answered) Yes Dirt Magnesium Carbonate In Water Magnesium carbonate is generally recognized as a white, solid compound that is insoluble in water but readily dissolves in acids. Also small amounts of magnesium carbonate (about 6% of the magnesium supplied by rivers) are deposited with calcite (caco3) in seawater. Magnesium carbonate is an inorganic salt with the chemical formula mgco3. Students react magnesium sulfate and sodium carbonate to. Magnesium Carbonate In Water.
From www.degruyter.com
Synthesis of magnesium carbonate hydrate from natural talc Magnesium Carbonate In Water The anhydrous salt is practically insoluble in water, acetone, and ammonia. Magnesium carbonate is an inorganic salt with the chemical formula mgco3. All forms of magnesium carbonate dissolve in acids. Magnesium carbonate is used as an antacid which reduces stomach acid. Students react magnesium sulfate and sodium carbonate to form magnesium carbonate, which is insoluble in water. Also small amounts. Magnesium Carbonate In Water.
From www.dreamstime.com
Magnesium Carbonate Molecule Stock Vector Illustration of Magnesium Carbonate In Water Includes kit list and safety instructions. Magnesium carbonate is generally recognized as a white, solid compound that is insoluble in water but readily dissolves in acids. Usually, this substance is positively identified through its chemical. All forms of magnesium carbonate dissolve in acids. Magnesium carbonate is used as an antacid which reduces stomach acid. There is little data for. There. Magnesium Carbonate In Water.
From www.sciencephoto.com
Magnesium carbonate in hydrochloric acid Stock Image A500/0601 Magnesium Carbonate In Water Includes kit list and safety instructions. The anhydrous salt is practically insoluble in water, acetone, and ammonia. Students react magnesium sulfate and sodium carbonate to form magnesium carbonate, which is insoluble in water. All forms of magnesium carbonate dissolve in acids. Also small amounts of magnesium carbonate (about 6% of the magnesium supplied by rivers) are deposited with calcite (caco3). Magnesium Carbonate In Water.
From www.healthydirections.com
Magnesium Carbonate What is it and How Does it Effect The Body Magnesium Carbonate In Water Magnesium carbonate is an inorganic salt with the chemical formula mgco3. When it comes to identifying magnesium carbonate, water solubility is just one of many properties that identify it. Also small amounts of magnesium carbonate (about 6% of the magnesium supplied by rivers) are deposited with calcite (caco3) in seawater. Magnesium carbonate, for example, has a solubility of about 0.02. Magnesium Carbonate In Water.
From www.chegg.com
Solved 1. Consider the insoluble salt magnesium carbonate in Magnesium Carbonate In Water All forms of magnesium carbonate dissolve in acids. Also small amounts of magnesium carbonate (about 6% of the magnesium supplied by rivers) are deposited with calcite (caco3) in seawater. Magnesium carbonate is used as an antacid which reduces stomach acid. Usually, this substance is positively identified through its chemical. Magnesium carbonate is generally recognized as a white, solid compound that. Magnesium Carbonate In Water.
From www.researchgate.net
(PDF) Hydrated Magnesium Carbonates, Formation and Stability in Ambient Magnesium Carbonate In Water Usually, this substance is positively identified through its chemical. Magnesium carbonate is used as an antacid which reduces stomach acid. There is significant uptake of. Magnesium carbonate is an inorganic salt with the chemical formula mgco3. When it comes to identifying magnesium carbonate, water solubility is just one of many properties that identify it. Students react magnesium sulfate and sodium. Magnesium Carbonate In Water.
From www.numerade.com
SOLVED Magnesium carbonate, MgCO3, is a salt of low solubility. When Magnesium Carbonate In Water Magnesium carbonate is used as an antacid which reduces stomach acid. The anhydrous salt is practically insoluble in water, acetone, and ammonia. Magnesium carbonate is an inorganic salt with the chemical formula mgco3. There is significant uptake of. Magnesium carbonate is generally recognized as a white, solid compound that is insoluble in water but readily dissolves in acids. Magnesium carbonate,. Magnesium Carbonate In Water.
From www.numerade.com
SOLVED Which ofthe following is the best representation of a mixture Magnesium Carbonate In Water When it comes to identifying magnesium carbonate, water solubility is just one of many properties that identify it. Includes kit list and safety instructions. Magnesium carbonate, for example, has a solubility of about 0.02 g per 100 g of water at room temperature. Magnesium carbonate is used as an antacid which reduces stomach acid. There is significant uptake of. Students. Magnesium Carbonate In Water.
From www.alibaba.com
Pure Magnesium Carbonate Pellets For Water With High Purity Buy Light Magnesium Carbonate In Water Usually, this substance is positively identified through its chemical. There is significant uptake of. Magnesium carbonate is generally recognized as a white, solid compound that is insoluble in water but readily dissolves in acids. Magnesium carbonate is used as an antacid which reduces stomach acid. Includes kit list and safety instructions. Magnesium carbonate is an inorganic salt with the chemical. Magnesium Carbonate In Water.
From www.numerade.com
SOLVED A geologist discovers a magnesium carbonate hydroxide hydrated Magnesium Carbonate In Water Students react magnesium sulfate and sodium carbonate to form magnesium carbonate, which is insoluble in water. Includes kit list and safety instructions. The anhydrous salt is practically insoluble in water, acetone, and ammonia. There is significant uptake of. Magnesium carbonate, for example, has a solubility of about 0.02 g per 100 g of water at room temperature. All forms of. Magnesium Carbonate In Water.
From www.youtube.com
Reaction between Magnesium and Water (Mg + H2O) YouTube Magnesium Carbonate In Water There is little data for. Magnesium carbonate is used as an antacid which reduces stomach acid. There is significant uptake of. All forms of magnesium carbonate dissolve in acids. Magnesium carbonate is generally recognized as a white, solid compound that is insoluble in water but readily dissolves in acids. When it comes to identifying magnesium carbonate, water solubility is just. Magnesium Carbonate In Water.
From www.researchgate.net
(PDF) Synthesis of magnesium carbonate hydrate from natural talc Magnesium Carbonate In Water Magnesium carbonate is generally recognized as a white, solid compound that is insoluble in water but readily dissolves in acids. Includes kit list and safety instructions. When it comes to identifying magnesium carbonate, water solubility is just one of many properties that identify it. Magnesium carbonate is used as an antacid which reduces stomach acid. Magnesium carbonate is an inorganic. Magnesium Carbonate In Water.
From www.fireworkscookbook.com
Magnesium Carbonate Fireworks Cookbook Magnesium Carbonate In Water There is little data for. Students react magnesium sulfate and sodium carbonate to form magnesium carbonate, which is insoluble in water. Usually, this substance is positively identified through its chemical. Magnesium carbonate is generally recognized as a white, solid compound that is insoluble in water but readily dissolves in acids. Also small amounts of magnesium carbonate (about 6% of the. Magnesium Carbonate In Water.
From www.tradekorea.com
Magnesium Carbonate tradekorea Magnesium Carbonate In Water All forms of magnesium carbonate dissolve in acids. Includes kit list and safety instructions. There is significant uptake of. Magnesium carbonate is an inorganic salt with the chemical formula mgco3. Magnesium carbonate is generally recognized as a white, solid compound that is insoluble in water but readily dissolves in acids. Magnesium carbonate is used as an antacid which reduces stomach. Magnesium Carbonate In Water.
From www.wakeupbodynutrition.com
Magnesium Carbonate in Liquid Chalk Magnesia 250 ml (improve your grip Magnesium Carbonate In Water Usually, this substance is positively identified through its chemical. All forms of magnesium carbonate dissolve in acids. There is significant uptake of. There is little data for. Magnesium carbonate is an inorganic salt with the chemical formula mgco3. When it comes to identifying magnesium carbonate, water solubility is just one of many properties that identify it. Magnesium carbonate, for example,. Magnesium Carbonate In Water.
From www.chegg.com
Solved of magnesium carbonate produces Magnesium Carbonate In Water Also small amounts of magnesium carbonate (about 6% of the magnesium supplied by rivers) are deposited with calcite (caco3) in seawater. The anhydrous salt is practically insoluble in water, acetone, and ammonia. All forms of magnesium carbonate dissolve in acids. Magnesium carbonate is generally recognized as a white, solid compound that is insoluble in water but readily dissolves in acids.. Magnesium Carbonate In Water.
From www.youtube.com
Magnesium Bicarbonate Water (How I Make It) YouTube Magnesium Carbonate In Water Students react magnesium sulfate and sodium carbonate to form magnesium carbonate, which is insoluble in water. All forms of magnesium carbonate dissolve in acids. Usually, this substance is positively identified through its chemical. Magnesium carbonate is used as an antacid which reduces stomach acid. Magnesium carbonate, for example, has a solubility of about 0.02 g per 100 g of water. Magnesium Carbonate In Water.
From www.hbarsci.com
Light Magnesium Carbonate, 100g The Curated Chemical Collection — hBARSCI Magnesium Carbonate In Water Also small amounts of magnesium carbonate (about 6% of the magnesium supplied by rivers) are deposited with calcite (caco3) in seawater. Magnesium carbonate is an inorganic salt with the chemical formula mgco3. There is significant uptake of. Includes kit list and safety instructions. When it comes to identifying magnesium carbonate, water solubility is just one of many properties that identify. Magnesium Carbonate In Water.
From www.youtube.com
The molar solubility of magnesium carbonate is 1.87x10^4 mol/L Magnesium Carbonate In Water When it comes to identifying magnesium carbonate, water solubility is just one of many properties that identify it. There is significant uptake of. Usually, this substance is positively identified through its chemical. All forms of magnesium carbonate dissolve in acids. Includes kit list and safety instructions. Magnesium carbonate is an inorganic salt with the chemical formula mgco3. There is little. Magnesium Carbonate In Water.
From www.garrisonminerals.com
Magnesium Carbonate Garrison Minerals Magnesium Carbonate In Water There is significant uptake of. Students react magnesium sulfate and sodium carbonate to form magnesium carbonate, which is insoluble in water. Also small amounts of magnesium carbonate (about 6% of the magnesium supplied by rivers) are deposited with calcite (caco3) in seawater. Includes kit list and safety instructions. Usually, this substance is positively identified through its chemical. Magnesium carbonate is. Magnesium Carbonate In Water.
From www.researchgate.net
SEM microphotographs of the magnesium carbonate hydrates prepared at Magnesium Carbonate In Water The anhydrous salt is practically insoluble in water, acetone, and ammonia. There is little data for. Magnesium carbonate, for example, has a solubility of about 0.02 g per 100 g of water at room temperature. Magnesium carbonate is generally recognized as a white, solid compound that is insoluble in water but readily dissolves in acids. Magnesium carbonate is used as. Magnesium Carbonate In Water.
From edu.rsc.org
Making magnesium carbonate the formation of an insoluble salt in water Magnesium Carbonate In Water Usually, this substance is positively identified through its chemical. Magnesium carbonate, for example, has a solubility of about 0.02 g per 100 g of water at room temperature. Magnesium carbonate is used as an antacid which reduces stomach acid. Also small amounts of magnesium carbonate (about 6% of the magnesium supplied by rivers) are deposited with calcite (caco3) in seawater.. Magnesium Carbonate In Water.
From www.alamy.com
Magnesite, MgCO3 (magnesium carbonate Stock Photo Alamy Magnesium Carbonate In Water Magnesium carbonate, for example, has a solubility of about 0.02 g per 100 g of water at room temperature. Students react magnesium sulfate and sodium carbonate to form magnesium carbonate, which is insoluble in water. When it comes to identifying magnesium carbonate, water solubility is just one of many properties that identify it. There is little data for. There is. Magnesium Carbonate In Water.
From sciencekitstore.com
Magnesium Carbonate, Basic, USP, "Extra Light" (32 fl oz or 950 cc Magnesium Carbonate In Water There is little data for. Magnesium carbonate is used as an antacid which reduces stomach acid. There is significant uptake of. The anhydrous salt is practically insoluble in water, acetone, and ammonia. Usually, this substance is positively identified through its chemical. Magnesium carbonate is generally recognized as a white, solid compound that is insoluble in water but readily dissolves in. Magnesium Carbonate In Water.
From www.pinterest.com
Making magnesium carbonate the formation of an insoluble salt in water Magnesium Carbonate In Water There is significant uptake of. Magnesium carbonate is used as an antacid which reduces stomach acid. There is little data for. The anhydrous salt is practically insoluble in water, acetone, and ammonia. Magnesium carbonate is an inorganic salt with the chemical formula mgco3. Usually, this substance is positively identified through its chemical. Students react magnesium sulfate and sodium carbonate to. Magnesium Carbonate In Water.
From setarvan.com
MAGNESIUM CARBONATE Magnesium Carbonate In Water Magnesium carbonate, for example, has a solubility of about 0.02 g per 100 g of water at room temperature. When it comes to identifying magnesium carbonate, water solubility is just one of many properties that identify it. Includes kit list and safety instructions. Usually, this substance is positively identified through its chemical. Magnesium carbonate is an inorganic salt with the. Magnesium Carbonate In Water.
From www.sciencephoto.com
Magnesium carbonate precipitate Stock Image C028/0739 Science Magnesium Carbonate In Water Usually, this substance is positively identified through its chemical. There is little data for. The anhydrous salt is practically insoluble in water, acetone, and ammonia. Magnesium carbonate, for example, has a solubility of about 0.02 g per 100 g of water at room temperature. Includes kit list and safety instructions. There is significant uptake of. Students react magnesium sulfate and. Magnesium Carbonate In Water.
From mavink.com
Magnesium Reacts With Hydrochloric Acid Magnesium Carbonate In Water Magnesium carbonate, for example, has a solubility of about 0.02 g per 100 g of water at room temperature. Includes kit list and safety instructions. The anhydrous salt is practically insoluble in water, acetone, and ammonia. Usually, this substance is positively identified through its chemical. Also small amounts of magnesium carbonate (about 6% of the magnesium supplied by rivers) are. Magnesium Carbonate In Water.
From naturalcalm.ca
Magnesium Carbonate Your Guide to This Type of Magnesium Supplement Magnesium Carbonate In Water Students react magnesium sulfate and sodium carbonate to form magnesium carbonate, which is insoluble in water. Magnesium carbonate is generally recognized as a white, solid compound that is insoluble in water but readily dissolves in acids. There is significant uptake of. Magnesium carbonate is used as an antacid which reduces stomach acid. Usually, this substance is positively identified through its. Magnesium Carbonate In Water.
From ar.inspiredpencil.com
Magnesium And Water Magnesium Carbonate In Water All forms of magnesium carbonate dissolve in acids. Magnesium carbonate is used as an antacid which reduces stomach acid. Magnesium carbonate, for example, has a solubility of about 0.02 g per 100 g of water at room temperature. When it comes to identifying magnesium carbonate, water solubility is just one of many properties that identify it. Magnesium carbonate is an. Magnesium Carbonate In Water.
From www.numerade.com
SOLVED The molar solubility of magnesium carbonate in a water solution Magnesium Carbonate In Water Students react magnesium sulfate and sodium carbonate to form magnesium carbonate, which is insoluble in water. There is little data for. Magnesium carbonate is an inorganic salt with the chemical formula mgco3. Magnesium carbonate, for example, has a solubility of about 0.02 g per 100 g of water at room temperature. Magnesium carbonate is generally recognized as a white, solid. Magnesium Carbonate In Water.
From www.ecplaza.net
Magnesium Carbonate Nanjing Jiayi Sunway Chemical Co.,Ltd Magnesium Carbonate In Water Also small amounts of magnesium carbonate (about 6% of the magnesium supplied by rivers) are deposited with calcite (caco3) in seawater. There is significant uptake of. There is little data for. Magnesium carbonate is used as an antacid which reduces stomach acid. The anhydrous salt is practically insoluble in water, acetone, and ammonia. Magnesium carbonate is an inorganic salt with. Magnesium Carbonate In Water.