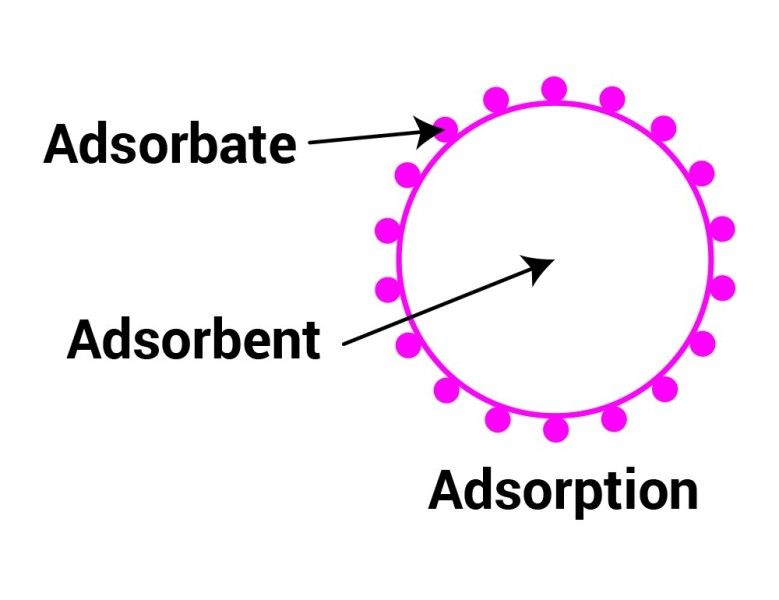
Define Base Adsorption . Adsorption refers to the process of adhesion of molecules or ions to a solid surface. Adsorption and absorption are two sorption processes through which one substance attaches to another. Ba = weight of base/ weight of solid. It is the phenomenon where substances from a fluid phase, such as gases or liquids, accumulate on the. Base adsorption factor (ba) base adsorption is defined as the number of grams of liquid base required to produce the capsulated mixture when mixed with 1 gm of solid(s). • m/g factor is used mostly in. The main difference between them is that adsorption is the. Adsorption can be used to treat waste streams or purify valuable components of a feed. • ba is number of grams of liquid base required to produce a capsule mixture with 1 gram of solid. Adsorption is a process whereby a substance (adsorbate, or sorbate) is accumulated on the surface of a solid (adsorbent, or sorbent).
from studiousguy.com
Ba = weight of base/ weight of solid. • m/g factor is used mostly in. Adsorption and absorption are two sorption processes through which one substance attaches to another. Adsorption is a process whereby a substance (adsorbate, or sorbate) is accumulated on the surface of a solid (adsorbent, or sorbent). Base adsorption factor (ba) base adsorption is defined as the number of grams of liquid base required to produce the capsulated mixture when mixed with 1 gm of solid(s). It is the phenomenon where substances from a fluid phase, such as gases or liquids, accumulate on the. • ba is number of grams of liquid base required to produce a capsule mixture with 1 gram of solid. The main difference between them is that adsorption is the. Adsorption can be used to treat waste streams or purify valuable components of a feed. Adsorption refers to the process of adhesion of molecules or ions to a solid surface.
8 Examples of Adsorption in Daily Life StudiousGuy
Define Base Adsorption The main difference between them is that adsorption is the. It is the phenomenon where substances from a fluid phase, such as gases or liquids, accumulate on the. Adsorption refers to the process of adhesion of molecules or ions to a solid surface. Adsorption can be used to treat waste streams or purify valuable components of a feed. Adsorption is a process whereby a substance (adsorbate, or sorbate) is accumulated on the surface of a solid (adsorbent, or sorbent). Adsorption and absorption are two sorption processes through which one substance attaches to another. • m/g factor is used mostly in. • ba is number of grams of liquid base required to produce a capsule mixture with 1 gram of solid. The main difference between them is that adsorption is the. Ba = weight of base/ weight of solid. Base adsorption factor (ba) base adsorption is defined as the number of grams of liquid base required to produce the capsulated mixture when mixed with 1 gm of solid(s).
From www.slideserve.com
PPT ADSORPTION PowerPoint Presentation, free download ID1757925 Define Base Adsorption • m/g factor is used mostly in. Adsorption and absorption are two sorption processes through which one substance attaches to another. Adsorption refers to the process of adhesion of molecules or ions to a solid surface. Adsorption can be used to treat waste streams or purify valuable components of a feed. • ba is number of grams of liquid base. Define Base Adsorption.
From pt.slideshare.net
Adsorption Define Base Adsorption Adsorption can be used to treat waste streams or purify valuable components of a feed. It is the phenomenon where substances from a fluid phase, such as gases or liquids, accumulate on the. • ba is number of grams of liquid base required to produce a capsule mixture with 1 gram of solid. Adsorption is a process whereby a substance. Define Base Adsorption.
From pubs.acs.org
Can TemperatureProgrammed Techniques Provide the Gold Standard for Define Base Adsorption Ba = weight of base/ weight of solid. Adsorption is a process whereby a substance (adsorbate, or sorbate) is accumulated on the surface of a solid (adsorbent, or sorbent). Adsorption and absorption are two sorption processes through which one substance attaches to another. Base adsorption factor (ba) base adsorption is defined as the number of grams of liquid base required. Define Base Adsorption.
From www.slideserve.com
PPT ADSORPTION PowerPoint Presentation, free download ID9085876 Define Base Adsorption The main difference between them is that adsorption is the. Adsorption and absorption are two sorption processes through which one substance attaches to another. Adsorption can be used to treat waste streams or purify valuable components of a feed. Base adsorption factor (ba) base adsorption is defined as the number of grams of liquid base required to produce the capsulated. Define Base Adsorption.
From learncheme.com
adsorptionconceptests LearnChemE Define Base Adsorption Adsorption can be used to treat waste streams or purify valuable components of a feed. Adsorption and absorption are two sorption processes through which one substance attaches to another. Ba = weight of base/ weight of solid. • ba is number of grams of liquid base required to produce a capsule mixture with 1 gram of solid. • m/g factor. Define Base Adsorption.
From www.dreamstime.com
Difference between Adsorption and Absorption Vector Illustration Stock Define Base Adsorption • ba is number of grams of liquid base required to produce a capsule mixture with 1 gram of solid. • m/g factor is used mostly in. Adsorption and absorption are two sorption processes through which one substance attaches to another. Base adsorption factor (ba) base adsorption is defined as the number of grams of liquid base required to produce. Define Base Adsorption.
From jobsforsustainability.com
Adsorption Process Development Engineer at Svante Define Base Adsorption Adsorption is a process whereby a substance (adsorbate, or sorbate) is accumulated on the surface of a solid (adsorbent, or sorbent). Ba = weight of base/ weight of solid. • m/g factor is used mostly in. Adsorption and absorption are two sorption processes through which one substance attaches to another. Base adsorption factor (ba) base adsorption is defined as the. Define Base Adsorption.
From ppt-online.org
Physical chemistry of surface phenomena. Basics of adsorptive therapy Define Base Adsorption Adsorption is a process whereby a substance (adsorbate, or sorbate) is accumulated on the surface of a solid (adsorbent, or sorbent). Base adsorption factor (ba) base adsorption is defined as the number of grams of liquid base required to produce the capsulated mixture when mixed with 1 gm of solid(s). The main difference between them is that adsorption is the.. Define Base Adsorption.
From www.youtube.com
Adsorption Isotherms And Their Types YouTube Define Base Adsorption Ba = weight of base/ weight of solid. Adsorption refers to the process of adhesion of molecules or ions to a solid surface. The main difference between them is that adsorption is the. Adsorption and absorption are two sorption processes through which one substance attaches to another. Adsorption can be used to treat waste streams or purify valuable components of. Define Base Adsorption.
From www.youtube.com
Adsorption Vs Absorption (Differences) YouTube Define Base Adsorption Adsorption is a process whereby a substance (adsorbate, or sorbate) is accumulated on the surface of a solid (adsorbent, or sorbent). Adsorption and absorption are two sorption processes through which one substance attaches to another. Adsorption can be used to treat waste streams or purify valuable components of a feed. • ba is number of grams of liquid base required. Define Base Adsorption.
From www.youtube.com
Define adsorption. YouTube Define Base Adsorption The main difference between them is that adsorption is the. • ba is number of grams of liquid base required to produce a capsule mixture with 1 gram of solid. Base adsorption factor (ba) base adsorption is defined as the number of grams of liquid base required to produce the capsulated mixture when mixed with 1 gm of solid(s). Adsorption. Define Base Adsorption.
From www.slideserve.com
PPT Adsorption Equilibrium PowerPoint Presentation, free download Define Base Adsorption Base adsorption factor (ba) base adsorption is defined as the number of grams of liquid base required to produce the capsulated mixture when mixed with 1 gm of solid(s). Adsorption and absorption are two sorption processes through which one substance attaches to another. Adsorption is a process whereby a substance (adsorbate, or sorbate) is accumulated on the surface of a. Define Base Adsorption.
From slidetodoc.com
Adsorption and Mineral Surface Reactions Lecture 24 Adsorption Define Base Adsorption Adsorption and absorption are two sorption processes through which one substance attaches to another. The main difference between them is that adsorption is the. It is the phenomenon where substances from a fluid phase, such as gases or liquids, accumulate on the. Adsorption is a process whereby a substance (adsorbate, or sorbate) is accumulated on the surface of a solid. Define Base Adsorption.
From chemistnotes.com
Adsorption Isotherm and Its Types Chemistry Notes Define Base Adsorption Adsorption is a process whereby a substance (adsorbate, or sorbate) is accumulated on the surface of a solid (adsorbent, or sorbent). • m/g factor is used mostly in. Base adsorption factor (ba) base adsorption is defined as the number of grams of liquid base required to produce the capsulated mixture when mixed with 1 gm of solid(s). Adsorption refers to. Define Base Adsorption.
From www.researchgate.net
Adsorption and desorption on the active sites of a heterogeneous Define Base Adsorption Base adsorption factor (ba) base adsorption is defined as the number of grams of liquid base required to produce the capsulated mixture when mixed with 1 gm of solid(s). • ba is number of grams of liquid base required to produce a capsule mixture with 1 gram of solid. Adsorption and absorption are two sorption processes through which one substance. Define Base Adsorption.
From www.youtube.com
TYPES OF ADSORPTION ISOTHERMS YouTube Define Base Adsorption Ba = weight of base/ weight of solid. Adsorption can be used to treat waste streams or purify valuable components of a feed. Adsorption and absorption are two sorption processes through which one substance attaches to another. • ba is number of grams of liquid base required to produce a capsule mixture with 1 gram of solid. • m/g factor. Define Base Adsorption.
From biodifferences.net
Difference between Absorption and Adsorption Bio Differences Define Base Adsorption • ba is number of grams of liquid base required to produce a capsule mixture with 1 gram of solid. Adsorption can be used to treat waste streams or purify valuable components of a feed. Ba = weight of base/ weight of solid. Adsorption and absorption are two sorption processes through which one substance attaches to another. Adsorption refers to. Define Base Adsorption.
From www.chegg.com
Solved a. Define the terms of chemical adsorption and Define Base Adsorption Ba = weight of base/ weight of solid. • ba is number of grams of liquid base required to produce a capsule mixture with 1 gram of solid. Adsorption is a process whereby a substance (adsorbate, or sorbate) is accumulated on the surface of a solid (adsorbent, or sorbent). The main difference between them is that adsorption is the. Adsorption. Define Base Adsorption.
From www.researchgate.net
Inhibition of nonspecific AuNP aggregation by dense thiol DNA Define Base Adsorption • m/g factor is used mostly in. The main difference between them is that adsorption is the. Adsorption refers to the process of adhesion of molecules or ions to a solid surface. Ba = weight of base/ weight of solid. It is the phenomenon where substances from a fluid phase, such as gases or liquids, accumulate on the. Adsorption is. Define Base Adsorption.
From www.mdpi.com
Processes Free FullText Adsorption and Desorption Behavior of Define Base Adsorption Adsorption can be used to treat waste streams or purify valuable components of a feed. Base adsorption factor (ba) base adsorption is defined as the number of grams of liquid base required to produce the capsulated mixture when mixed with 1 gm of solid(s). The main difference between them is that adsorption is the. Ba = weight of base/ weight. Define Base Adsorption.
From www.theengineersperspectives.com
Elution vs. Adsorption The Engineer's Perspective Define Base Adsorption Adsorption and absorption are two sorption processes through which one substance attaches to another. • ba is number of grams of liquid base required to produce a capsule mixture with 1 gram of solid. Adsorption can be used to treat waste streams or purify valuable components of a feed. Ba = weight of base/ weight of solid. • m/g factor. Define Base Adsorption.
From www.researchgate.net
(A) The double stranded structure of DNA. (B) The DNA backbone Define Base Adsorption • m/g factor is used mostly in. It is the phenomenon where substances from a fluid phase, such as gases or liquids, accumulate on the. Adsorption refers to the process of adhesion of molecules or ions to a solid surface. Adsorption and absorption are two sorption processes through which one substance attaches to another. Adsorption can be used to treat. Define Base Adsorption.
From www.aquaportail.com
Absorption définition et explications AquaPortail Define Base Adsorption Adsorption and absorption are two sorption processes through which one substance attaches to another. Adsorption is a process whereby a substance (adsorbate, or sorbate) is accumulated on the surface of a solid (adsorbent, or sorbent). • m/g factor is used mostly in. Adsorption can be used to treat waste streams or purify valuable components of a feed. It is the. Define Base Adsorption.
From www.researchgate.net
a N2 adsorptiondesorption isotherms and b pore size distribution Define Base Adsorption The main difference between them is that adsorption is the. • m/g factor is used mostly in. Adsorption can be used to treat waste streams or purify valuable components of a feed. It is the phenomenon where substances from a fluid phase, such as gases or liquids, accumulate on the. • ba is number of grams of liquid base required. Define Base Adsorption.
From www.researchgate.net
Schematic representation of the adsorption mechanism of antibiotics Define Base Adsorption • ba is number of grams of liquid base required to produce a capsule mixture with 1 gram of solid. Adsorption and absorption are two sorption processes through which one substance attaches to another. Adsorption is a process whereby a substance (adsorbate, or sorbate) is accumulated on the surface of a solid (adsorbent, or sorbent). • m/g factor is used. Define Base Adsorption.
From www.youtube.com
Base adsorption and minim per gram factor(M/g) for soft gelatin Define Base Adsorption Adsorption and absorption are two sorption processes through which one substance attaches to another. Adsorption refers to the process of adhesion of molecules or ions to a solid surface. The main difference between them is that adsorption is the. Adsorption can be used to treat waste streams or purify valuable components of a feed. It is the phenomenon where substances. Define Base Adsorption.
From www.alamyimages.fr
Différence entre l'adsorption et l'absorption. Absorption une Define Base Adsorption It is the phenomenon where substances from a fluid phase, such as gases or liquids, accumulate on the. • m/g factor is used mostly in. The main difference between them is that adsorption is the. Adsorption can be used to treat waste streams or purify valuable components of a feed. Adsorption and absorption are two sorption processes through which one. Define Base Adsorption.
From sciencenotes.org
Adsorption vs Absorption Differences and Examples Define Base Adsorption Base adsorption factor (ba) base adsorption is defined as the number of grams of liquid base required to produce the capsulated mixture when mixed with 1 gm of solid(s). Adsorption is a process whereby a substance (adsorbate, or sorbate) is accumulated on the surface of a solid (adsorbent, or sorbent). Ba = weight of base/ weight of solid. • m/g. Define Base Adsorption.
From netsolwater.com
What distinguishes adsorption from absorption Define Base Adsorption Adsorption refers to the process of adhesion of molecules or ions to a solid surface. • ba is number of grams of liquid base required to produce a capsule mixture with 1 gram of solid. Ba = weight of base/ weight of solid. Base adsorption factor (ba) base adsorption is defined as the number of grams of liquid base required. Define Base Adsorption.
From www.researchgate.net
The adsorption mechanism of FCCA aerogel beads on TC. The adsorption Define Base Adsorption Adsorption is a process whereby a substance (adsorbate, or sorbate) is accumulated on the surface of a solid (adsorbent, or sorbent). Adsorption refers to the process of adhesion of molecules or ions to a solid surface. • ba is number of grams of liquid base required to produce a capsule mixture with 1 gram of solid. The main difference between. Define Base Adsorption.
From adsorptioninformationformat.com
Adsorption Information Format a universal adsorption format Define Base Adsorption Adsorption and absorption are two sorption processes through which one substance attaches to another. • m/g factor is used mostly in. Base adsorption factor (ba) base adsorption is defined as the number of grams of liquid base required to produce the capsulated mixture when mixed with 1 gm of solid(s). • ba is number of grams of liquid base required. Define Base Adsorption.
From www.doubtnut.com
Define adsorption. Write any two features which distinguish physisorpt Define Base Adsorption Adsorption can be used to treat waste streams or purify valuable components of a feed. • m/g factor is used mostly in. • ba is number of grams of liquid base required to produce a capsule mixture with 1 gram of solid. Adsorption refers to the process of adhesion of molecules or ions to a solid surface. It is the. Define Base Adsorption.
From studiousguy.com
8 Examples of Adsorption in Daily Life StudiousGuy Define Base Adsorption Base adsorption factor (ba) base adsorption is defined as the number of grams of liquid base required to produce the capsulated mixture when mixed with 1 gm of solid(s). Adsorption can be used to treat waste streams or purify valuable components of a feed. • m/g factor is used mostly in. Adsorption and absorption are two sorption processes through which. Define Base Adsorption.
From www.doubtnut.com
Define adsorption. Write any two features which distinguish physisorpt Define Base Adsorption Adsorption can be used to treat waste streams or purify valuable components of a feed. Adsorption refers to the process of adhesion of molecules or ions to a solid surface. • m/g factor is used mostly in. Base adsorption factor (ba) base adsorption is defined as the number of grams of liquid base required to produce the capsulated mixture when. Define Base Adsorption.
From www.youtube.com
LECTURE7 APPLICATION OF ADSORPTION YouTube Define Base Adsorption Base adsorption factor (ba) base adsorption is defined as the number of grams of liquid base required to produce the capsulated mixture when mixed with 1 gm of solid(s). It is the phenomenon where substances from a fluid phase, such as gases or liquids, accumulate on the. Adsorption can be used to treat waste streams or purify valuable components of. Define Base Adsorption.