Iron Iii Chloride And Sodium Carbonate . If the metal can form ions with different charges, a roman numeral in parentheses follows the name of the metal to specify its charge. Mixing iron (iii) chloride and sodium carbonate solutions results in forming of a brownish precipitate with the liberation of a. This video is the practical demonstration of the reaction of iron(iii) chloride (fecl3) with. It only uses iron metal and sodium carbonate, which are readily available. The balanced equation will be calculated along with the. To react with 0.00225 moles of iron (iii) chloride, 0.00225 ⋅ 3 2 = 0.003375 moles of sodium carbonate is needed. The result is an orange precipitate. The hexaaquairon(iii) ion is sufficiently acidic to react with the weakly basic carbonate ion. Iron(iii) ions and carbonate ions. Thus, fecl 2 is iron(ii) chloride and fecl 3 is iron(iii). Sodium bicarbonate (baking soda) can be used instead. Here, sodium carbonate (na 2 co 3) is added to iron (iii) ion (fe 3+). Enter an equation of an ionic chemical equation and press the balance button.
from solvedlib.com
The balanced equation will be calculated along with the. The hexaaquairon(iii) ion is sufficiently acidic to react with the weakly basic carbonate ion. Sodium bicarbonate (baking soda) can be used instead. To react with 0.00225 moles of iron (iii) chloride, 0.00225 ⋅ 3 2 = 0.003375 moles of sodium carbonate is needed. It only uses iron metal and sodium carbonate, which are readily available. Mixing iron (iii) chloride and sodium carbonate solutions results in forming of a brownish precipitate with the liberation of a. The result is an orange precipitate. If the metal can form ions with different charges, a roman numeral in parentheses follows the name of the metal to specify its charge. Here, sodium carbonate (na 2 co 3) is added to iron (iii) ion (fe 3+). This video is the practical demonstration of the reaction of iron(iii) chloride (fecl3) with.
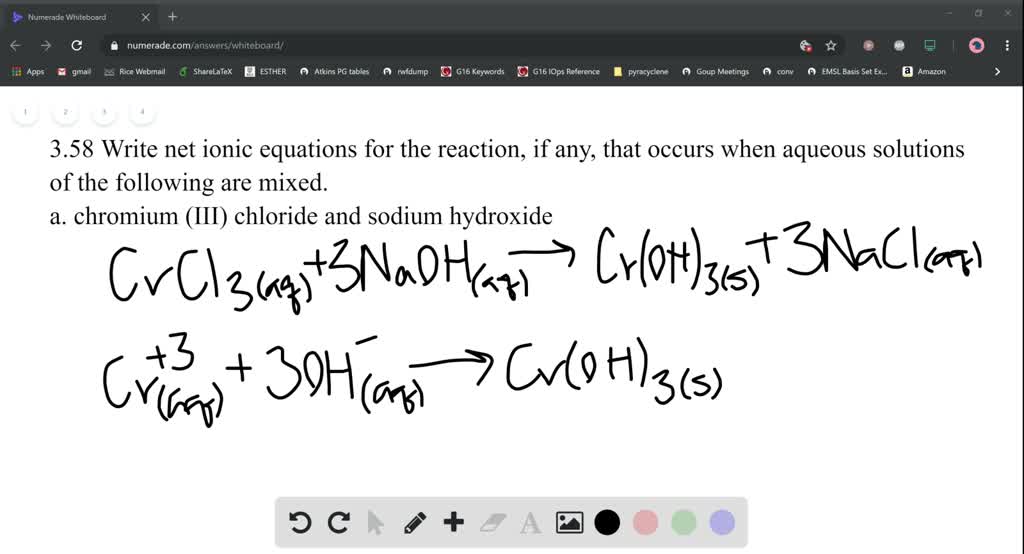
Write net ionic equations for the reaction, if any, t… SolvedLib
Iron Iii Chloride And Sodium Carbonate To react with 0.00225 moles of iron (iii) chloride, 0.00225 ⋅ 3 2 = 0.003375 moles of sodium carbonate is needed. Enter an equation of an ionic chemical equation and press the balance button. Iron(iii) ions and carbonate ions. The hexaaquairon(iii) ion is sufficiently acidic to react with the weakly basic carbonate ion. The balanced equation will be calculated along with the. Thus, fecl 2 is iron(ii) chloride and fecl 3 is iron(iii). Sodium bicarbonate (baking soda) can be used instead. Here, sodium carbonate (na 2 co 3) is added to iron (iii) ion (fe 3+). This video is the practical demonstration of the reaction of iron(iii) chloride (fecl3) with. Mixing iron (iii) chloride and sodium carbonate solutions results in forming of a brownish precipitate with the liberation of a. It only uses iron metal and sodium carbonate, which are readily available. The result is an orange precipitate. To react with 0.00225 moles of iron (iii) chloride, 0.00225 ⋅ 3 2 = 0.003375 moles of sodium carbonate is needed. If the metal can form ions with different charges, a roman numeral in parentheses follows the name of the metal to specify its charge.
From www.youtube.com
Sodium Carbonate and Calcium Chloride Reaction YouTube Iron Iii Chloride And Sodium Carbonate Here, sodium carbonate (na 2 co 3) is added to iron (iii) ion (fe 3+). It only uses iron metal and sodium carbonate, which are readily available. The result is an orange precipitate. The hexaaquairon(iii) ion is sufficiently acidic to react with the weakly basic carbonate ion. Enter an equation of an ionic chemical equation and press the balance button.. Iron Iii Chloride And Sodium Carbonate.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Iron Iii Chloride And Sodium Carbonate Sodium bicarbonate (baking soda) can be used instead. This video is the practical demonstration of the reaction of iron(iii) chloride (fecl3) with. Mixing iron (iii) chloride and sodium carbonate solutions results in forming of a brownish precipitate with the liberation of a. Enter an equation of an ionic chemical equation and press the balance button. The result is an orange. Iron Iii Chloride And Sodium Carbonate.
From hedycabrera.blogspot.com
25+ Calcium Hydroxide And Hydrochloric Acid Ionic Equation Images Iron Iii Chloride And Sodium Carbonate Here, sodium carbonate (na 2 co 3) is added to iron (iii) ion (fe 3+). Thus, fecl 2 is iron(ii) chloride and fecl 3 is iron(iii). The hexaaquairon(iii) ion is sufficiently acidic to react with the weakly basic carbonate ion. Sodium bicarbonate (baking soda) can be used instead. It only uses iron metal and sodium carbonate, which are readily available.. Iron Iii Chloride And Sodium Carbonate.
From www.youtube.com
Iron III Chloride Reaction With Potassium Thiocyanate (FeCl3 + KSCN Iron Iii Chloride And Sodium Carbonate This video is the practical demonstration of the reaction of iron(iii) chloride (fecl3) with. The hexaaquairon(iii) ion is sufficiently acidic to react with the weakly basic carbonate ion. It only uses iron metal and sodium carbonate, which are readily available. To react with 0.00225 moles of iron (iii) chloride, 0.00225 ⋅ 3 2 = 0.003375 moles of sodium carbonate is. Iron Iii Chloride And Sodium Carbonate.
From www.youtube.com
Sodium carbonate and Hydrochloric acid (HCl) reaction !Chemistry Iron Iii Chloride And Sodium Carbonate Mixing iron (iii) chloride and sodium carbonate solutions results in forming of a brownish precipitate with the liberation of a. Here, sodium carbonate (na 2 co 3) is added to iron (iii) ion (fe 3+). If the metal can form ions with different charges, a roman numeral in parentheses follows the name of the metal to specify its charge. The. Iron Iii Chloride And Sodium Carbonate.
From www.youtube.com
Addition sodium hydroxide to iron (II) chloride and iron (III) chloride Iron Iii Chloride And Sodium Carbonate The hexaaquairon(iii) ion is sufficiently acidic to react with the weakly basic carbonate ion. The result is an orange precipitate. This video is the practical demonstration of the reaction of iron(iii) chloride (fecl3) with. To react with 0.00225 moles of iron (iii) chloride, 0.00225 ⋅ 3 2 = 0.003375 moles of sodium carbonate is needed. Sodium bicarbonate (baking soda) can. Iron Iii Chloride And Sodium Carbonate.
From www.youtube.com
Double displacement of FeCl3 + Na2CO3 Ferric chloride + Sodium Iron Iii Chloride And Sodium Carbonate The balanced equation will be calculated along with the. The result is an orange precipitate. It only uses iron metal and sodium carbonate, which are readily available. Thus, fecl 2 is iron(ii) chloride and fecl 3 is iron(iii). Here, sodium carbonate (na 2 co 3) is added to iron (iii) ion (fe 3+). Sodium bicarbonate (baking soda) can be used. Iron Iii Chloride And Sodium Carbonate.
From lauriekyrran.blogspot.com
Potassium Hydroxide And Iron Ii Nitrate Precipitate LaurieKyrran Iron Iii Chloride And Sodium Carbonate This video is the practical demonstration of the reaction of iron(iii) chloride (fecl3) with. Iron(iii) ions and carbonate ions. The balanced equation will be calculated along with the. Mixing iron (iii) chloride and sodium carbonate solutions results in forming of a brownish precipitate with the liberation of a. To react with 0.00225 moles of iron (iii) chloride, 0.00225 ⋅ 3. Iron Iii Chloride And Sodium Carbonate.
From www.youtube.com
Reaction between sodium carbonate and calcium chloride YouTube Iron Iii Chloride And Sodium Carbonate The hexaaquairon(iii) ion is sufficiently acidic to react with the weakly basic carbonate ion. To react with 0.00225 moles of iron (iii) chloride, 0.00225 ⋅ 3 2 = 0.003375 moles of sodium carbonate is needed. Mixing iron (iii) chloride and sodium carbonate solutions results in forming of a brownish precipitate with the liberation of a. Here, sodium carbonate (na 2. Iron Iii Chloride And Sodium Carbonate.
From www.teachoo.com
Case Based Class 10 Science The physical states of the reactants Iron Iii Chloride And Sodium Carbonate To react with 0.00225 moles of iron (iii) chloride, 0.00225 ⋅ 3 2 = 0.003375 moles of sodium carbonate is needed. Sodium bicarbonate (baking soda) can be used instead. If the metal can form ions with different charges, a roman numeral in parentheses follows the name of the metal to specify its charge. It only uses iron metal and sodium. Iron Iii Chloride And Sodium Carbonate.
From stock.adobe.com
Chemical reactionPipette 0.25 M solution of iron(III) chloride (FeCl3 Iron Iii Chloride And Sodium Carbonate To react with 0.00225 moles of iron (iii) chloride, 0.00225 ⋅ 3 2 = 0.003375 moles of sodium carbonate is needed. The result is an orange precipitate. Sodium bicarbonate (baking soda) can be used instead. Iron(iii) ions and carbonate ions. If the metal can form ions with different charges, a roman numeral in parentheses follows the name of the metal. Iron Iii Chloride And Sodium Carbonate.
From www.bartleby.com
Answered When aqueous solutions of iron(II)… bartleby Iron Iii Chloride And Sodium Carbonate The balanced equation will be calculated along with the. Thus, fecl 2 is iron(ii) chloride and fecl 3 is iron(iii). It only uses iron metal and sodium carbonate, which are readily available. The result is an orange precipitate. The hexaaquairon(iii) ion is sufficiently acidic to react with the weakly basic carbonate ion. Sodium bicarbonate (baking soda) can be used instead.. Iron Iii Chloride And Sodium Carbonate.
From chem.libretexts.org
5.6 Day 41 Electrolysis; Commercial Batteries Chemistry LibreTexts Iron Iii Chloride And Sodium Carbonate To react with 0.00225 moles of iron (iii) chloride, 0.00225 ⋅ 3 2 = 0.003375 moles of sodium carbonate is needed. Mixing iron (iii) chloride and sodium carbonate solutions results in forming of a brownish precipitate with the liberation of a. Sodium bicarbonate (baking soda) can be used instead. The balanced equation will be calculated along with the. Here, sodium. Iron Iii Chloride And Sodium Carbonate.
From www.alamy.com
Iron (III) hydroxide precipitation. Test tube containing sodium Iron Iii Chloride And Sodium Carbonate The result is an orange precipitate. This video is the practical demonstration of the reaction of iron(iii) chloride (fecl3) with. It only uses iron metal and sodium carbonate, which are readily available. The hexaaquairon(iii) ion is sufficiently acidic to react with the weakly basic carbonate ion. Thus, fecl 2 is iron(ii) chloride and fecl 3 is iron(iii). The balanced equation. Iron Iii Chloride And Sodium Carbonate.
From solvedlib.com
Write net ionic equations for the reaction, if any, t… SolvedLib Iron Iii Chloride And Sodium Carbonate The balanced equation will be calculated along with the. It only uses iron metal and sodium carbonate, which are readily available. Iron(iii) ions and carbonate ions. Enter an equation of an ionic chemical equation and press the balance button. Here, sodium carbonate (na 2 co 3) is added to iron (iii) ion (fe 3+). The result is an orange precipitate.. Iron Iii Chloride And Sodium Carbonate.
From www.youtube.com
How to Write the Net Ionic Equation for FeCl2 + Na2CO3 = FeCO3 + NaCl Iron Iii Chloride And Sodium Carbonate Here, sodium carbonate (na 2 co 3) is added to iron (iii) ion (fe 3+). Iron(iii) ions and carbonate ions. The result is an orange precipitate. Sodium bicarbonate (baking soda) can be used instead. To react with 0.00225 moles of iron (iii) chloride, 0.00225 ⋅ 3 2 = 0.003375 moles of sodium carbonate is needed. Enter an equation of an. Iron Iii Chloride And Sodium Carbonate.
From www.bartleby.com
Answered When aqueous solutions of iron(II)… bartleby Iron Iii Chloride And Sodium Carbonate Enter an equation of an ionic chemical equation and press the balance button. The result is an orange precipitate. This video is the practical demonstration of the reaction of iron(iii) chloride (fecl3) with. To react with 0.00225 moles of iron (iii) chloride, 0.00225 ⋅ 3 2 = 0.003375 moles of sodium carbonate is needed. It only uses iron metal and. Iron Iii Chloride And Sodium Carbonate.
From stock.adobe.com
Chemical reactionRusty red iron(III) hydroxide precipitate (Fe(OH)3 Iron Iii Chloride And Sodium Carbonate The result is an orange precipitate. Mixing iron (iii) chloride and sodium carbonate solutions results in forming of a brownish precipitate with the liberation of a. If the metal can form ions with different charges, a roman numeral in parentheses follows the name of the metal to specify its charge. It only uses iron metal and sodium carbonate, which are. Iron Iii Chloride And Sodium Carbonate.
From www.numerade.com
SOLVED The mass of iron(II) carbonate that is dissolved in 125 mL of a Iron Iii Chloride And Sodium Carbonate The result is an orange precipitate. Mixing iron (iii) chloride and sodium carbonate solutions results in forming of a brownish precipitate with the liberation of a. Iron(iii) ions and carbonate ions. Here, sodium carbonate (na 2 co 3) is added to iron (iii) ion (fe 3+). Thus, fecl 2 is iron(ii) chloride and fecl 3 is iron(iii). It only uses. Iron Iii Chloride And Sodium Carbonate.
From www.slideserve.com
PPT How are salts made and named? PowerPoint Presentation ID5348558 Iron Iii Chloride And Sodium Carbonate Thus, fecl 2 is iron(ii) chloride and fecl 3 is iron(iii). The balanced equation will be calculated along with the. Mixing iron (iii) chloride and sodium carbonate solutions results in forming of a brownish precipitate with the liberation of a. It only uses iron metal and sodium carbonate, which are readily available. Here, sodium carbonate (na 2 co 3) is. Iron Iii Chloride And Sodium Carbonate.
From www.youtube.com
Calcium Chloride with Sodium Carbonate YouTube Iron Iii Chloride And Sodium Carbonate It only uses iron metal and sodium carbonate, which are readily available. Thus, fecl 2 is iron(ii) chloride and fecl 3 is iron(iii). To react with 0.00225 moles of iron (iii) chloride, 0.00225 ⋅ 3 2 = 0.003375 moles of sodium carbonate is needed. Sodium bicarbonate (baking soda) can be used instead. Mixing iron (iii) chloride and sodium carbonate solutions. Iron Iii Chloride And Sodium Carbonate.
From www.youtube.com
How to Write the Formula for Iron (III) nitrate YouTube Iron Iii Chloride And Sodium Carbonate The result is an orange precipitate. Mixing iron (iii) chloride and sodium carbonate solutions results in forming of a brownish precipitate with the liberation of a. It only uses iron metal and sodium carbonate, which are readily available. The balanced equation will be calculated along with the. If the metal can form ions with different charges, a roman numeral in. Iron Iii Chloride And Sodium Carbonate.
From www.reddit.com
Precipitate Colors of Metal Ions in Aqueous Ammonia and Sodium Iron Iii Chloride And Sodium Carbonate The hexaaquairon(iii) ion is sufficiently acidic to react with the weakly basic carbonate ion. The balanced equation will be calculated along with the. Here, sodium carbonate (na 2 co 3) is added to iron (iii) ion (fe 3+). The result is an orange precipitate. This video is the practical demonstration of the reaction of iron(iii) chloride (fecl3) with. Thus, fecl. Iron Iii Chloride And Sodium Carbonate.
From www.slideserve.com
PPT Prentice Hall Chemistry (c) 2005 PowerPoint Presentation ID6658952 Iron Iii Chloride And Sodium Carbonate Thus, fecl 2 is iron(ii) chloride and fecl 3 is iron(iii). Iron(iii) ions and carbonate ions. It only uses iron metal and sodium carbonate, which are readily available. To react with 0.00225 moles of iron (iii) chloride, 0.00225 ⋅ 3 2 = 0.003375 moles of sodium carbonate is needed. If the metal can form ions with different charges, a roman. Iron Iii Chloride And Sodium Carbonate.
From insende.netlify.app
30+ Hydrochloric Acid And Sodium Hydroxide Equation Insende Iron Iii Chloride And Sodium Carbonate Here, sodium carbonate (na 2 co 3) is added to iron (iii) ion (fe 3+). Sodium bicarbonate (baking soda) can be used instead. This video is the practical demonstration of the reaction of iron(iii) chloride (fecl3) with. Iron(iii) ions and carbonate ions. Mixing iron (iii) chloride and sodium carbonate solutions results in forming of a brownish precipitate with the liberation. Iron Iii Chloride And Sodium Carbonate.
From www.nagwa.com
Question Video Identifying a Series of Methods to Separate Sodium Iron Iii Chloride And Sodium Carbonate The balanced equation will be calculated along with the. It only uses iron metal and sodium carbonate, which are readily available. Here, sodium carbonate (na 2 co 3) is added to iron (iii) ion (fe 3+). Iron(iii) ions and carbonate ions. If the metal can form ions with different charges, a roman numeral in parentheses follows the name of the. Iron Iii Chloride And Sodium Carbonate.
From www.numerade.com
SOLVED Sodium carbonate reacts with hydrochloric acid to form sodium Iron Iii Chloride And Sodium Carbonate Iron(iii) ions and carbonate ions. It only uses iron metal and sodium carbonate, which are readily available. Sodium bicarbonate (baking soda) can be used instead. The result is an orange precipitate. If the metal can form ions with different charges, a roman numeral in parentheses follows the name of the metal to specify its charge. This video is the practical. Iron Iii Chloride And Sodium Carbonate.
From www.chegg.com
Solved Iron(III) chloride + Potassium phosphate Yellowbrown Iron Iii Chloride And Sodium Carbonate Sodium bicarbonate (baking soda) can be used instead. This video is the practical demonstration of the reaction of iron(iii) chloride (fecl3) with. To react with 0.00225 moles of iron (iii) chloride, 0.00225 ⋅ 3 2 = 0.003375 moles of sodium carbonate is needed. The balanced equation will be calculated along with the. If the metal can form ions with different. Iron Iii Chloride And Sodium Carbonate.
From ocdrum.com
nickel(ii iodide and potassium carbonate balanced equation) Iron Iii Chloride And Sodium Carbonate To react with 0.00225 moles of iron (iii) chloride, 0.00225 ⋅ 3 2 = 0.003375 moles of sodium carbonate is needed. The balanced equation will be calculated along with the. Here, sodium carbonate (na 2 co 3) is added to iron (iii) ion (fe 3+). This video is the practical demonstration of the reaction of iron(iii) chloride (fecl3) with. Thus,. Iron Iii Chloride And Sodium Carbonate.
From www.shimico.com
Sodium carbonate and the methods of production Shimico blog Iron Iii Chloride And Sodium Carbonate Enter an equation of an ionic chemical equation and press the balance button. If the metal can form ions with different charges, a roman numeral in parentheses follows the name of the metal to specify its charge. It only uses iron metal and sodium carbonate, which are readily available. To react with 0.00225 moles of iron (iii) chloride, 0.00225 ⋅. Iron Iii Chloride And Sodium Carbonate.
From brainly.in
aqueous iron chloride and sodium carbonate solution yields aqueous Iron Iii Chloride And Sodium Carbonate Sodium bicarbonate (baking soda) can be used instead. If the metal can form ions with different charges, a roman numeral in parentheses follows the name of the metal to specify its charge. Iron(iii) ions and carbonate ions. To react with 0.00225 moles of iron (iii) chloride, 0.00225 ⋅ 3 2 = 0.003375 moles of sodium carbonate is needed. Enter an. Iron Iii Chloride And Sodium Carbonate.
From duncan-jolpblogtravis.blogspot.com
Iron Iii Nitrate and Sodium Carbonate Balanced Equation Iron Iii Chloride And Sodium Carbonate The balanced equation will be calculated along with the. Here, sodium carbonate (na 2 co 3) is added to iron (iii) ion (fe 3+). To react with 0.00225 moles of iron (iii) chloride, 0.00225 ⋅ 3 2 = 0.003375 moles of sodium carbonate is needed. If the metal can form ions with different charges, a roman numeral in parentheses follows. Iron Iii Chloride And Sodium Carbonate.
From www.slideserve.com
PPT Oral assessment PowerPoint Presentation, free download ID3444733 Iron Iii Chloride And Sodium Carbonate This video is the practical demonstration of the reaction of iron(iii) chloride (fecl3) with. The result is an orange precipitate. To react with 0.00225 moles of iron (iii) chloride, 0.00225 ⋅ 3 2 = 0.003375 moles of sodium carbonate is needed. Mixing iron (iii) chloride and sodium carbonate solutions results in forming of a brownish precipitate with the liberation of. Iron Iii Chloride And Sodium Carbonate.
From tyrelldesnhlawson.blogspot.com
Ammonium Chloride and Sodium Hydroxide Net Ionic Equation Iron Iii Chloride And Sodium Carbonate It only uses iron metal and sodium carbonate, which are readily available. Sodium bicarbonate (baking soda) can be used instead. Thus, fecl 2 is iron(ii) chloride and fecl 3 is iron(iii). The result is an orange precipitate. The balanced equation will be calculated along with the. If the metal can form ions with different charges, a roman numeral in parentheses. Iron Iii Chloride And Sodium Carbonate.
From davis-well-rodgers.blogspot.com
Iron Iii Chloride and Sodium Hydroxide Ionic Equation DaviswellRodgers Iron Iii Chloride And Sodium Carbonate Here, sodium carbonate (na 2 co 3) is added to iron (iii) ion (fe 3+). Sodium bicarbonate (baking soda) can be used instead. It only uses iron metal and sodium carbonate, which are readily available. The balanced equation will be calculated along with the. To react with 0.00225 moles of iron (iii) chloride, 0.00225 ⋅ 3 2 = 0.003375 moles. Iron Iii Chloride And Sodium Carbonate.