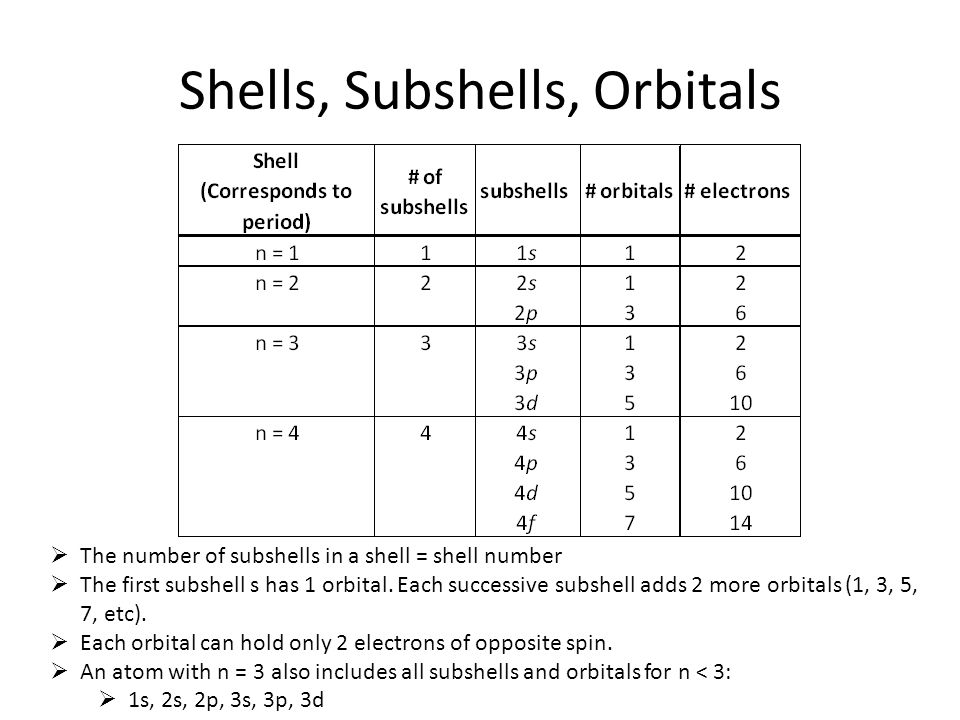
Subshell Vs Sublevel . All electrons that have the same value for n n (the principle quantum number) are in the same shell. Summary of the arrangement of electrons in atoms. Each shell has a different energy level, increasing the further it is from the nucleus. Within a shell (same n n), all electrons that share the same l l (the angular. Orbitals with the same value of l form a subshell. When writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the. The principal quantum shells increase in energy with. Orbitals exist at specific energy levels and electrons can only be found at these. Electrons orbit the nucleus of an atom at different ranges, called shells. The subshells contain one or more atomic orbitals. Subshell is the pathway in which an electron. Shell is the pathway followed by electrons around an atom’s nucleus. In addition, the greater the angular momentum quantum number, the greater is the angular momentum of an electron at this orbital. Orbitals with l = 0 are called s orbitals (or the s subshells).
from lavelle.chem.ucla.edu
Orbitals with l = 0 are called s orbitals (or the s subshells). Summary of the arrangement of electrons in atoms. The principal quantum shells increase in energy with. In addition, the greater the angular momentum quantum number, the greater is the angular momentum of an electron at this orbital. Orbitals exist at specific energy levels and electrons can only be found at these. Subshell is the pathway in which an electron. All electrons that have the same value for n n (the principle quantum number) are in the same shell. The subshells contain one or more atomic orbitals. Each shell has a different energy level, increasing the further it is from the nucleus. Shell is the pathway followed by electrons around an atom’s nucleus.
subshells and orbitals CHEMISTRY COMMUNITY
Subshell Vs Sublevel When writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the. All electrons that have the same value for n n (the principle quantum number) are in the same shell. Orbitals exist at specific energy levels and electrons can only be found at these. In addition, the greater the angular momentum quantum number, the greater is the angular momentum of an electron at this orbital. Subshell is the pathway in which an electron. Summary of the arrangement of electrons in atoms. The principal quantum shells increase in energy with. Orbitals with the same value of l form a subshell. Orbitals with l = 0 are called s orbitals (or the s subshells). Each shell has a different energy level, increasing the further it is from the nucleus. Within a shell (same n n), all electrons that share the same l l (the angular. Electrons orbit the nucleus of an atom at different ranges, called shells. When writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the. Shell is the pathway followed by electrons around an atom’s nucleus. The subshells contain one or more atomic orbitals.
From www.coscinecreative.com
What is the Difference in a Shell, Subshell and Orbital? — CoScine Creative Subshell Vs Sublevel The subshells contain one or more atomic orbitals. The principal quantum shells increase in energy with. Electrons orbit the nucleus of an atom at different ranges, called shells. Summary of the arrangement of electrons in atoms. All electrons that have the same value for n n (the principle quantum number) are in the same shell. Subshell is the pathway in. Subshell Vs Sublevel.
From www.youtube.com
What is the difference between a shell, a subshell and an orbital Subshell Vs Sublevel When writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the. Subshell is the pathway in which an electron. Within a shell (same n n), all electrons that share the same l l (the angular. Summary of the arrangement of electrons in atoms. Orbitals exist at specific. Subshell Vs Sublevel.
From sciencing.com
Energy Level Definition, Equation (w/ Diagrams) Sciencing Subshell Vs Sublevel The subshells contain one or more atomic orbitals. Shell is the pathway followed by electrons around an atom’s nucleus. All electrons that have the same value for n n (the principle quantum number) are in the same shell. In addition, the greater the angular momentum quantum number, the greater is the angular momentum of an electron at this orbital. Summary. Subshell Vs Sublevel.
From www.youtube.com
Energy Level Shells Subshells Orbitals Bohr's Formula Easy Subshell Vs Sublevel Electrons orbit the nucleus of an atom at different ranges, called shells. Orbitals exist at specific energy levels and electrons can only be found at these. Within a shell (same n n), all electrons that share the same l l (the angular. In addition, the greater the angular momentum quantum number, the greater is the angular momentum of an electron. Subshell Vs Sublevel.
From chemistryskills.com
Shapes of Orbitals and their Types Chemistry Skills Subshell Vs Sublevel Each shell has a different energy level, increasing the further it is from the nucleus. Electrons orbit the nucleus of an atom at different ranges, called shells. The principal quantum shells increase in energy with. In addition, the greater the angular momentum quantum number, the greater is the angular momentum of an electron at this orbital. Orbitals with the same. Subshell Vs Sublevel.
From pressbooks.bccampus.ca
8.3 Development of Quantum Theory CHEM 1114 Introduction to Chemistry Subshell Vs Sublevel All electrons that have the same value for n n (the principle quantum number) are in the same shell. In addition, the greater the angular momentum quantum number, the greater is the angular momentum of an electron at this orbital. When writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the. Subshell Vs Sublevel.
From schematicdiagramtelium.z13.web.core.windows.net
Orbital Diagram For Se Subshell Vs Sublevel Orbitals exist at specific energy levels and electrons can only be found at these. The subshells contain one or more atomic orbitals. In addition, the greater the angular momentum quantum number, the greater is the angular momentum of an electron at this orbital. All electrons that have the same value for n n (the principle quantum number) are in the. Subshell Vs Sublevel.
From courses.lumenlearning.com
Development of Quantum Theory Chemistry I Subshell Vs Sublevel All electrons that have the same value for n n (the principle quantum number) are in the same shell. Orbitals with l = 0 are called s orbitals (or the s subshells). In addition, the greater the angular momentum quantum number, the greater is the angular momentum of an electron at this orbital. The subshells contain one or more atomic. Subshell Vs Sublevel.
From mungfali.com
Electron Subshell Chart Subshell Vs Sublevel Orbitals exist at specific energy levels and electrons can only be found at these. Orbitals with the same value of l form a subshell. All electrons that have the same value for n n (the principle quantum number) are in the same shell. Shell is the pathway followed by electrons around an atom’s nucleus. The principal quantum shells increase in. Subshell Vs Sublevel.
From www.youtube.com
Energy levels, sublevels, & orbitals YouTube Subshell Vs Sublevel Electrons orbit the nucleus of an atom at different ranges, called shells. The principal quantum shells increase in energy with. Subshell is the pathway in which an electron. Within a shell (same n n), all electrons that share the same l l (the angular. Orbitals exist at specific energy levels and electrons can only be found at these. Orbitals with. Subshell Vs Sublevel.
From www.youtube.com
Science Tutorial Writing the Electron Configuration Subshell Notation Subshell Vs Sublevel The principal quantum shells increase in energy with. Orbitals exist at specific energy levels and electrons can only be found at these. Electrons orbit the nucleus of an atom at different ranges, called shells. Each shell has a different energy level, increasing the further it is from the nucleus. The subshells contain one or more atomic orbitals. All electrons that. Subshell Vs Sublevel.
From www.youtube.com
What is the Difference between Shell and Subshell Chemistry Class 9 Subshell Vs Sublevel Electrons orbit the nucleus of an atom at different ranges, called shells. In addition, the greater the angular momentum quantum number, the greater is the angular momentum of an electron at this orbital. The subshells contain one or more atomic orbitals. Within a shell (same n n), all electrons that share the same l l (the angular. Subshell is the. Subshell Vs Sublevel.
From readingandwritingprojectcom.web.fc2.com
how many orbitals are in the n = 3 level? Subshell Vs Sublevel The principal quantum shells increase in energy with. All electrons that have the same value for n n (the principle quantum number) are in the same shell. In addition, the greater the angular momentum quantum number, the greater is the angular momentum of an electron at this orbital. Electrons orbit the nucleus of an atom at different ranges, called shells.. Subshell Vs Sublevel.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps Subshell Vs Sublevel Orbitals with the same value of l form a subshell. The principal quantum shells increase in energy with. When writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the. Shell is the pathway followed by electrons around an atom’s nucleus. All electrons that have the same value. Subshell Vs Sublevel.
From www.slideshare.net
Chemistry Electron orbitals and sub levels Subshell Vs Sublevel Summary of the arrangement of electrons in atoms. Orbitals exist at specific energy levels and electrons can only be found at these. Shell is the pathway followed by electrons around an atom’s nucleus. Subshell is the pathway in which an electron. In addition, the greater the angular momentum quantum number, the greater is the angular momentum of an electron at. Subshell Vs Sublevel.
From thecontentauthority.com
Subshell vs Sublevel Which Should You Use In Writing? Subshell Vs Sublevel Orbitals with the same value of l form a subshell. Orbitals with l = 0 are called s orbitals (or the s subshells). The subshells contain one or more atomic orbitals. Shell is the pathway followed by electrons around an atom’s nucleus. Electrons orbit the nucleus of an atom at different ranges, called shells. In addition, the greater the angular. Subshell Vs Sublevel.
From thecontentauthority.com
Subshell vs Orbital How Are These Words Connected? Subshell Vs Sublevel When writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the. The subshells contain one or more atomic orbitals. All electrons that have the same value for n n (the principle quantum number) are in the same shell. Orbitals with l = 0 are called s orbitals. Subshell Vs Sublevel.
From pediaa.com
Difference Between Shell Subshell and Orbital Definition, Structure Subshell Vs Sublevel The principal quantum shells increase in energy with. Summary of the arrangement of electrons in atoms. In addition, the greater the angular momentum quantum number, the greater is the angular momentum of an electron at this orbital. Within a shell (same n n), all electrons that share the same l l (the angular. All electrons that have the same value. Subshell Vs Sublevel.
From lavelle.chem.ucla.edu
subshells and orbitals CHEMISTRY COMMUNITY Subshell Vs Sublevel All electrons that have the same value for n n (the principle quantum number) are in the same shell. Orbitals exist at specific energy levels and electrons can only be found at these. Orbitals with l = 0 are called s orbitals (or the s subshells). Orbitals with the same value of l form a subshell. The subshells contain one. Subshell Vs Sublevel.
From www.techgamingreport.com
A Brief Guide on Electron Configuration Calculator Subshell Vs Sublevel Orbitals with l = 0 are called s orbitals (or the s subshells). In addition, the greater the angular momentum quantum number, the greater is the angular momentum of an electron at this orbital. Orbitals with the same value of l form a subshell. The principal quantum shells increase in energy with. Electrons orbit the nucleus of an atom at. Subshell Vs Sublevel.
From www.youtube.com
Shells, Subshells, and Orbitals l Understand the difference YouTube Subshell Vs Sublevel Each shell has a different energy level, increasing the further it is from the nucleus. In addition, the greater the angular momentum quantum number, the greater is the angular momentum of an electron at this orbital. Orbitals with the same value of l form a subshell. Orbitals with l = 0 are called s orbitals (or the s subshells). Summary. Subshell Vs Sublevel.
From ar.inspiredpencil.com
Electron Subshell Subshell Vs Sublevel When writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the. Orbitals exist at specific energy levels and electrons can only be found at these. Orbitals with the same value of l form a subshell. Subshell is the pathway in which an electron. The subshells contain one. Subshell Vs Sublevel.
From www.sliderbase.com
Energy Levels, Sublevels, Electrons Subshell Vs Sublevel Each shell has a different energy level, increasing the further it is from the nucleus. Electrons orbit the nucleus of an atom at different ranges, called shells. Orbitals with l = 0 are called s orbitals (or the s subshells). Shell is the pathway followed by electrons around an atom’s nucleus. Subshell is the pathway in which an electron. In. Subshell Vs Sublevel.
From www.hanlin.com
AQA A Level Chemistry复习笔记1.1.5 Electron Configuration翰林国际教育 Subshell Vs Sublevel When writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the. The subshells contain one or more atomic orbitals. Within a shell (same n n), all electrons that share the same l l (the angular. Orbitals exist at specific energy levels and electrons can only be found. Subshell Vs Sublevel.
From learn.careers360.com
What is the difference between orbit and orbital? Subshell Vs Sublevel Within a shell (same n n), all electrons that share the same l l (the angular. Orbitals with the same value of l form a subshell. The subshells contain one or more atomic orbitals. All electrons that have the same value for n n (the principle quantum number) are in the same shell. When writing an electron configuration, first write. Subshell Vs Sublevel.
From www.savemyexams.co.uk
Electron Configuration (1.1.5) AQA A Level Chemistry Revision Notes Subshell Vs Sublevel Orbitals exist at specific energy levels and electrons can only be found at these. The subshells contain one or more atomic orbitals. All electrons that have the same value for n n (the principle quantum number) are in the same shell. When writing an electron configuration, first write the energy level (the period), then the subshell to be filled and. Subshell Vs Sublevel.
From unacademy.com
Notes on the Irregularites of Electronic Configuration of Transition Metals Subshell Vs Sublevel Orbitals exist at specific energy levels and electrons can only be found at these. Within a shell (same n n), all electrons that share the same l l (the angular. Electrons orbit the nucleus of an atom at different ranges, called shells. Summary of the arrangement of electrons in atoms. All electrons that have the same value for n n. Subshell Vs Sublevel.
From h-o-m-e.org
Chemistry Sublevels An Introduction to s, p, d, and f Sublevels Subshell Vs Sublevel When writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the. Subshell is the pathway in which an electron. Each shell has a different energy level, increasing the further it is from the nucleus. Electrons orbit the nucleus of an atom at different ranges, called shells. All. Subshell Vs Sublevel.
From h-o-m-e.org
Chemistry Sublevels An Introduction to s, p, d, and f Sublevels Subshell Vs Sublevel Shell is the pathway followed by electrons around an atom’s nucleus. Orbitals with l = 0 are called s orbitals (or the s subshells). The subshells contain one or more atomic orbitals. All electrons that have the same value for n n (the principle quantum number) are in the same shell. Orbitals with the same value of l form a. Subshell Vs Sublevel.
From socratic.org
What is the difference between electron shells and electron orbitals Subshell Vs Sublevel In addition, the greater the angular momentum quantum number, the greater is the angular momentum of an electron at this orbital. The principal quantum shells increase in energy with. Within a shell (same n n), all electrons that share the same l l (the angular. The subshells contain one or more atomic orbitals. Each shell has a different energy level,. Subshell Vs Sublevel.
From schematicfixlankier.z21.web.core.windows.net
Al Orbital Diagram Subshell Vs Sublevel The principal quantum shells increase in energy with. Within a shell (same n n), all electrons that share the same l l (the angular. Subshell is the pathway in which an electron. Orbitals with the same value of l form a subshell. The subshells contain one or more atomic orbitals. Orbitals exist at specific energy levels and electrons can only. Subshell Vs Sublevel.
From diagrambomenslikpo.z13.web.core.windows.net
Understanding Molecular Orbital Diagrams Subshell Vs Sublevel Within a shell (same n n), all electrons that share the same l l (the angular. The principal quantum shells increase in energy with. Orbitals with l = 0 are called s orbitals (or the s subshells). Orbitals with the same value of l form a subshell. Orbitals exist at specific energy levels and electrons can only be found at. Subshell Vs Sublevel.
From chemistrymsq11.blogspot.com
Grade 11 CHAPTER 1 ATOMIC STRUCTURE SEMESTER1 Subshell Vs Sublevel Summary of the arrangement of electrons in atoms. When writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the. Subshell is the pathway in which an electron. All electrons that have the same value for n n (the principle quantum number) are in the same shell. Shell. Subshell Vs Sublevel.