Labeling Medical Devices . Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. Specifically, this document provides guidance on the content of the label, instructions for use, and information intended for the patient in order to. These regulations specify the minimum requirements for all devices. Design includes labeling content that. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Later sections in this chapter discuss any additional requirements.
from medicaldevicelicense.com
Specifically, this document provides guidance on the content of the label, instructions for use, and information intended for the patient in order to. These regulations specify the minimum requirements for all devices. Later sections in this chapter discuss any additional requirements. Design includes labeling content that. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling.
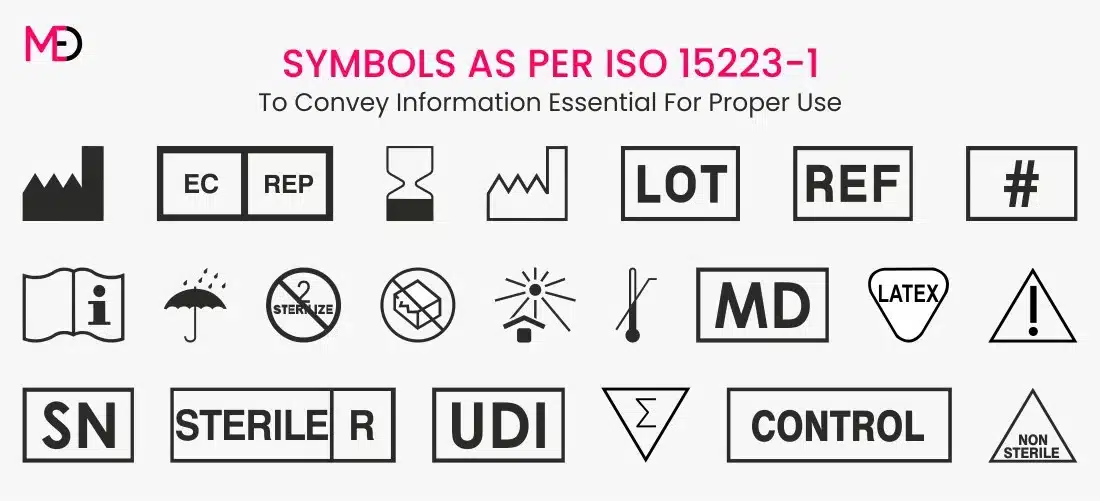
Essential Medical Device Symbols for Labeling ISO 152231
Labeling Medical Devices Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Specifically, this document provides guidance on the content of the label, instructions for use, and information intended for the patient in order to. Design includes labeling content that. Later sections in this chapter discuss any additional requirements. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. These regulations specify the minimum requirements for all devices.
From www.vrogue.co
Fda Medical Device Labeling Requirements Presentation vrogue.co Labeling Medical Devices Specifically, this document provides guidance on the content of the label, instructions for use, and information intended for the patient in order to. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Design includes labeling. Labeling Medical Devices.
From www.freseniusmedicalcare.com
MedizinprodukteVerordnung Fresenius Medical Care Labeling Medical Devices These regulations specify the minimum requirements for all devices. Design includes labeling content that. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Later sections in this chapter discuss any additional requirements. Specifically, this document. Labeling Medical Devices.
From www.greenlight.guru
FDA Medical Device Labeling Requirements An Overview Labeling Medical Devices These regulations specify the minimum requirements for all devices. Design includes labeling content that. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Specifically, this document provides guidance on the content of the label, instructions. Labeling Medical Devices.
From abr.com
Label Compliance AB&R® (American Barcode and RFID) Labeling Medical Devices Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Later sections in this chapter discuss any additional requirements. Specifically, this document provides guidance on the content of the label, instructions for use, and information intended for the patient in order to. These regulations specify the minimum requirements for all devices. Principles of labelling. Labeling Medical Devices.
From mavink.com
Medical Device Labeling Symbols Labeling Medical Devices Design includes labeling content that. These regulations specify the minimum requirements for all devices. Later sections in this chapter discuss any additional requirements. Specifically, this document provides guidance on the content of the label, instructions for use, and information intended for the patient in order to. Adequate labeling for a medical device requires proper design and procurement of the labels. Labeling Medical Devices.
From www.regdesk.co
FDA on General Principles of Labeling for Medical Devices RegDesk Labeling Medical Devices Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. Specifically, this document provides guidance on the content of the label, instructions for use, and information intended for the patient in order to. Later sections in. Labeling Medical Devices.
From mungfali.com
Medical Device Labeling Symbols Labeling Medical Devices Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. Specifically, this document provides guidance on the content of the label, instructions for use, and information intended for the patient in order to. These regulations specify the minimum requirements for all devices. Design includes labeling content that. Later sections in this chapter. Labeling Medical Devices.
From medicaldevicelicense.com
Essential Medical Device Symbols for Labeling ISO 152231 Labeling Medical Devices Later sections in this chapter discuss any additional requirements. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. Specifically, this document provides guidance on the content of the label, instructions for use, and information intended for the patient in order to. These regulations specify the minimum requirements for all devices. Design. Labeling Medical Devices.
From www.regdesk.co
HSA Guidance on UDI System Components and Labeling RegDesk Labeling Medical Devices Specifically, this document provides guidance on the content of the label, instructions for use, and information intended for the patient in order to. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Later sections in this chapter discuss any additional requirements. These regulations specify the minimum requirements for all devices. Principles of labelling. Labeling Medical Devices.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Labeling Medical Devices These regulations specify the minimum requirements for all devices. Specifically, this document provides guidance on the content of the label, instructions for use, and information intended for the patient in order to. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. Later sections in this chapter discuss any additional requirements. Adequate. Labeling Medical Devices.
From impactlabelling.ie
We design and print Medical Device Labels in Ireland Impact Labelling Labeling Medical Devices These regulations specify the minimum requirements for all devices. Design includes labeling content that. Later sections in this chapter discuss any additional requirements. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Specifically, this document provides guidance on the content of the label, instructions for use, and information intended for the patient in. Labeling Medical Devices.
From labelservice.co.uk
Medical Device Labels, Medical Device Labelling Labelservice Labeling Medical Devices These regulations specify the minimum requirements for all devices. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Design includes labeling content that. Later sections in this chapter discuss any additional requirements. Specifically, this document provides guidance on the content of the label, instructions for use, and information intended for the patient in. Labeling Medical Devices.
From www.vrogue.co
Medical Device Labeling Requirements What You Need To vrogue.co Labeling Medical Devices These regulations specify the minimum requirements for all devices. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Later sections in this chapter discuss any additional requirements. Specifically, this document provides guidance on the content of the label, instructions for use, and information intended for the patient in order to. Design includes labeling. Labeling Medical Devices.
From mungfali.com
Medical Device Labeling Symbols Labeling Medical Devices Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. Later sections in this chapter discuss any additional requirements. Specifically, this document provides guidance on the content of the label, instructions for use, and information intended for the patient in order to. These regulations specify the minimum requirements for all devices. Design. Labeling Medical Devices.
From exogphupj.blob.core.windows.net
Medical Device Labelling Tga at William Maurer blog Labeling Medical Devices Specifically, this document provides guidance on the content of the label, instructions for use, and information intended for the patient in order to. Design includes labeling content that. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. These regulations specify the minimum requirements for all devices. Later sections in this chapter. Labeling Medical Devices.
From easymedicaldevice.com
How to Create a Label as per EU MDR 2017/745? Labeling Medical Devices Specifically, this document provides guidance on the content of the label, instructions for use, and information intended for the patient in order to. Design includes labeling content that. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb). Labeling Medical Devices.
From vascufirst.com
What is the meaning of symbols on medical devices labels? VascuFirst Labeling Medical Devices These regulations specify the minimum requirements for all devices. Design includes labeling content that. Specifically, this document provides guidance on the content of the label, instructions for use, and information intended for the patient in order to. Later sections in this chapter discuss any additional requirements. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx. Labeling Medical Devices.
From clin-r.com
Labels for Medical Devices Clin R Labeling Medical Devices Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Later sections in this chapter discuss any additional requirements. These regulations specify the minimum requirements for all devices. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. Design includes labeling content that. Specifically, this document. Labeling Medical Devices.
From clin-r.com
Labels for Medical Devices Clin R Labeling Medical Devices Design includes labeling content that. Later sections in this chapter discuss any additional requirements. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. Specifically, this document provides guidance on the content of the label, instructions for use, and information intended for the patient in order to. Adequate labeling for a medical. Labeling Medical Devices.
From www.vrogue.co
Canadian Device Labeling Requirements Ce Mark Package vrogue.co Labeling Medical Devices Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. Later sections in this chapter discuss any additional requirements. Design includes labeling content that. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Specifically, this document provides guidance on the content of the label, instructions. Labeling Medical Devices.
From www.camcode.com
UDI Labels (Unique Device Identification) for Medical Devices Camcode Labeling Medical Devices Design includes labeling content that. Specifically, this document provides guidance on the content of the label, instructions for use, and information intended for the patient in order to. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Later sections in this chapter discuss any additional requirements. These regulations specify the minimum requirements for. Labeling Medical Devices.
From www.flexo-graphics.com
Medical Device Labeling Medical Equipment Labels Labeling Medical Devices Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Design includes labeling content that. Specifically, this document provides guidance on the content of the label, instructions for use, and information intended for the patient in order to. Later sections in this chapter discuss any additional requirements. Principles of labelling for medical devices and. Labeling Medical Devices.
From www.vrogue.co
How To Create A Label As Per Eu Mdr 2017745 2023 vrogue.co Labeling Medical Devices These regulations specify the minimum requirements for all devices. Design includes labeling content that. Later sections in this chapter discuss any additional requirements. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. Specifically, this document. Labeling Medical Devices.
From medenvoyglobal.com
Medical Device Labeling Requirements in Europe MedEnvoy Labeling Medical Devices Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. Specifically, this document provides guidance on the content of the label, instructions for use, and information intended for the patient in order to. These regulations specify the minimum requirements for all devices. Adequate labeling for a medical device requires proper design and. Labeling Medical Devices.
From www.regdesk.co
HSA Guidance on Labeling for Medical Devices Introduction RegDesk Labeling Medical Devices These regulations specify the minimum requirements for all devices. Design includes labeling content that. Later sections in this chapter discuss any additional requirements. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Specifically, this document. Labeling Medical Devices.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Labeling Medical Devices Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. Specifically, this document provides guidance on the content of the label, instructions for use, and information intended for the patient in order to. Design includes labeling. Labeling Medical Devices.
From vascufirst.com
What is the meaning of symbols on medical devices labels? VascuFirst Labeling Medical Devices Design includes labeling content that. Specifically, this document provides guidance on the content of the label, instructions for use, and information intended for the patient in order to. Later sections in this chapter discuss any additional requirements. These regulations specify the minimum requirements for all devices. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx. Labeling Medical Devices.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Labeling Medical Devices Later sections in this chapter discuss any additional requirements. These regulations specify the minimum requirements for all devices. Specifically, this document provides guidance on the content of the label, instructions for use, and information intended for the patient in order to. Design includes labeling content that. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx. Labeling Medical Devices.
From ambitiousmares.blogspot.com
31 Udi Label Examples Labels Design Ideas 2020 Labeling Medical Devices Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. These regulations specify the minimum requirements for all devices. Design includes labeling content that. Specifically, this document provides guidance on the content of the label, instructions. Labeling Medical Devices.
From onlinelibrary.wiley.com
Do Healthcare Professionals Comprehend Standardized Symbols Present on Labeling Medical Devices These regulations specify the minimum requirements for all devices. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Later sections in this chapter discuss any additional requirements. Design includes labeling content that. Specifically, this document. Labeling Medical Devices.
From www.schlafenderhase.com
A Guide to Medical Device Labeling Requirements Schlafender Hase Labeling Medical Devices Later sections in this chapter discuss any additional requirements. Specifically, this document provides guidance on the content of the label, instructions for use, and information intended for the patient in order to. Design includes labeling content that. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. These regulations specify the minimum. Labeling Medical Devices.
From exogphupj.blob.core.windows.net
Medical Device Labelling Tga at William Maurer blog Labeling Medical Devices Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. Specifically, this document provides guidance on the content of the label, instructions for use, and information intended for the patient in order to. Later sections in. Labeling Medical Devices.
From www.afpharmaservice.com
Medical Device Labelling Requirements Labeling Medical Devices Design includes labeling content that. These regulations specify the minimum requirements for all devices. Specifically, this document provides guidance on the content of the label, instructions for use, and information intended for the patient in order to. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. Adequate labeling for a medical. Labeling Medical Devices.
From old.sermitsiaq.ag
Medical Device Label Template Labeling Medical Devices Specifically, this document provides guidance on the content of the label, instructions for use, and information intended for the patient in order to. Adequate labeling for a medical device requires proper design and procurement of the labels and labeling. These regulations specify the minimum requirements for all devices. Later sections in this chapter discuss any additional requirements. Design includes labeling. Labeling Medical Devices.
From medicaldevicelicense.com
Medical Device Blog I Discover Latest Expert Analysis of MDR Labeling Medical Devices These regulations specify the minimum requirements for all devices. Specifically, this document provides guidance on the content of the label, instructions for use, and information intended for the patient in order to. Later sections in this chapter discuss any additional requirements. Principles of labelling for medical devices and ivd medical devices pdf (762.65 kb) docx (142.24 kb) member sites. Adequate. Labeling Medical Devices.