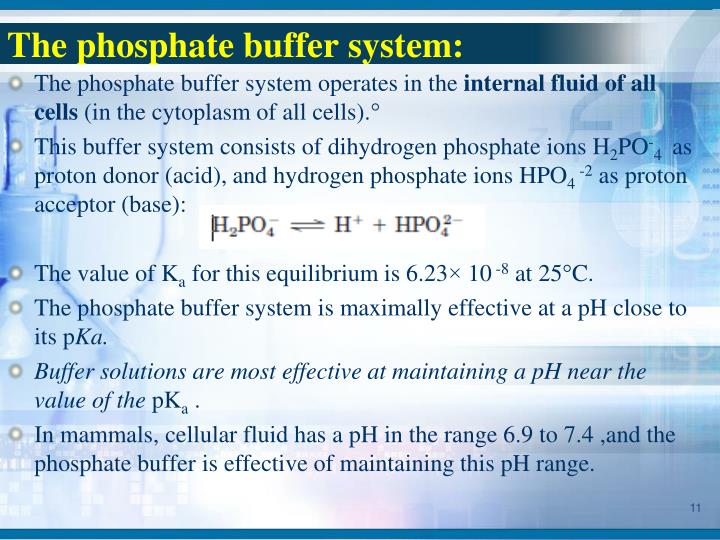
Use Pbs Buffer . The use of a physiological saline buffer is required for different purposes when working with nscs, mainly for tissue or cell. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. Phosphate buffers play a critical role in the food services industry in particular. Phosphate buffered saline (pbs) is a buffer solution commonly used in biological research. Learn how to better prepare and store your. By preparing 10x instead of 1x pbs solution, you can dilute 100ml of the 10x solution to 900ml of distilled water to obtain 1l of 1x pbs buffer. This technique is commonly used to test certain types of foods.
from www.slideserve.com
This technique is commonly used to test certain types of foods. Phosphate buffers play a critical role in the food services industry in particular. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. Learn how to better prepare and store your. Phosphate buffered saline (pbs) is a buffer solution commonly used in biological research. By preparing 10x instead of 1x pbs solution, you can dilute 100ml of the 10x solution to 900ml of distilled water to obtain 1l of 1x pbs buffer. The use of a physiological saline buffer is required for different purposes when working with nscs, mainly for tissue or cell.
PPT Buffers of Biological & Clinical Significance PowerPoint
Use Pbs Buffer The use of a physiological saline buffer is required for different purposes when working with nscs, mainly for tissue or cell. This technique is commonly used to test certain types of foods. Phosphate buffers play a critical role in the food services industry in particular. The use of a physiological saline buffer is required for different purposes when working with nscs, mainly for tissue or cell. Phosphate buffered saline (pbs) is a buffer solution commonly used in biological research. By preparing 10x instead of 1x pbs solution, you can dilute 100ml of the 10x solution to 900ml of distilled water to obtain 1l of 1x pbs buffer. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. Learn how to better prepare and store your.
From servicebioinstrument.en.made-in-china.com
10X Pbs Phosphate Buffered Saline Cell Culture Salt Solution Mammalian Use Pbs Buffer By preparing 10x instead of 1x pbs solution, you can dilute 100ml of the 10x solution to 900ml of distilled water to obtain 1l of 1x pbs buffer. Learn how to better prepare and store your. The use of a physiological saline buffer is required for different purposes when working with nscs, mainly for tissue or cell. Phosphate buffered saline. Use Pbs Buffer.
From www.labsolute-chemsolute.de
PBS Buffer Readytouse tablets and bags Th. Geyer Use Pbs Buffer This technique is commonly used to test certain types of foods. The use of a physiological saline buffer is required for different purposes when working with nscs, mainly for tissue or cell. Phosphate buffered saline (pbs) is a buffer solution commonly used in biological research. Phosphate buffers play a critical role in the food services industry in particular. By preparing. Use Pbs Buffer.
From www.chenyanglobal.com
Phosphate Buffered Saline Buffer, PBS A professional supplier of swabs Use Pbs Buffer Phosphate buffers play a critical role in the food services industry in particular. This technique is commonly used to test certain types of foods. By preparing 10x instead of 1x pbs solution, you can dilute 100ml of the 10x solution to 900ml of distilled water to obtain 1l of 1x pbs buffer. Learn how to better prepare and store your.. Use Pbs Buffer.
From animalia-life.club
Phosphate Buffer System Equation Use Pbs Buffer The use of a physiological saline buffer is required for different purposes when working with nscs, mainly for tissue or cell. This technique is commonly used to test certain types of foods. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. By preparing 10x instead of 1x. Use Pbs Buffer.
From www.fishersci.com
Invitrogen PBS PhosphateBuffered Saline (10X) pH 7.4, RNasefree 1L Use Pbs Buffer Phosphate buffers play a critical role in the food services industry in particular. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. By preparing 10x instead of 1x pbs solution, you can dilute 100ml of the 10x solution to 900ml of distilled water to obtain 1l of. Use Pbs Buffer.
From www.biomat.it
Phosphate buffer saline (PBS) Biomat Use Pbs Buffer Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. By preparing 10x instead of 1x pbs solution, you can dilute 100ml of the 10x solution to 900ml of distilled water to obtain 1l of 1x pbs buffer. Phosphate buffers play a critical role in the food services. Use Pbs Buffer.
From www.labsolute-chemsolute.com
PBS Buffer Readytouse tablets and bags Th. Geyer Use Pbs Buffer Learn how to better prepare and store your. This technique is commonly used to test certain types of foods. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. Phosphate buffers play a critical role in the food services industry in particular. Phosphate buffered saline (pbs) is a. Use Pbs Buffer.
From www.labsolute-chemsolute.com
PBS Buffer Readytouse tablets and bags Th. Geyer Use Pbs Buffer Phosphate buffers play a critical role in the food services industry in particular. Phosphate buffered saline (pbs) is a buffer solution commonly used in biological research. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. By preparing 10x instead of 1x pbs solution, you can dilute 100ml. Use Pbs Buffer.
From www.elabscience.com
ReadytoUse Phosphate Buffer (PBS) (10×) Designed to Support and Use Pbs Buffer Phosphate buffered saline (pbs) is a buffer solution commonly used in biological research. Learn how to better prepare and store your. This technique is commonly used to test certain types of foods. By preparing 10x instead of 1x pbs solution, you can dilute 100ml of the 10x solution to 900ml of distilled water to obtain 1l of 1x pbs buffer.. Use Pbs Buffer.
From www.genetoprotein.com
25X PBS Buffer Use Pbs Buffer Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. Phosphate buffers play a critical role in the food services industry in particular. Learn how to better prepare and store your. Phosphate buffered saline (pbs) is a buffer solution commonly used in biological research. This technique is commonly. Use Pbs Buffer.
From ecocyteshop.com
PBS Phosphate Buffered Saline (500 ml) Ready to use Use Pbs Buffer Phosphate buffered saline (pbs) is a buffer solution commonly used in biological research. Phosphate buffers play a critical role in the food services industry in particular. This technique is commonly used to test certain types of foods. The use of a physiological saline buffer is required for different purposes when working with nscs, mainly for tissue or cell. Buffers and. Use Pbs Buffer.
From www.deltamicroscopies.com
Phosphate Buffer Saline (PBS) Delta Microscopies Use Pbs Buffer Phosphate buffered saline (pbs) is a buffer solution commonly used in biological research. Phosphate buffers play a critical role in the food services industry in particular. By preparing 10x instead of 1x pbs solution, you can dilute 100ml of the 10x solution to 900ml of distilled water to obtain 1l of 1x pbs buffer. This technique is commonly used to. Use Pbs Buffer.
From www.deltamicroscopies.com
Dulbecco's Phosphate Buffer Saline DPBS Delta Microscopies Use Pbs Buffer Phosphate buffers play a critical role in the food services industry in particular. The use of a physiological saline buffer is required for different purposes when working with nscs, mainly for tissue or cell. By preparing 10x instead of 1x pbs solution, you can dilute 100ml of the 10x solution to 900ml of distilled water to obtain 1l of 1x. Use Pbs Buffer.
From advancedbiomatrix.com
Advanced BioMatrix Phosphate Buffered Saline (PBS) 10X 5076 Use Pbs Buffer Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. Phosphate buffers play a critical role in the food services industry in particular. This technique is commonly used to test certain types of foods. The use of a physiological saline buffer is required for different purposes when working. Use Pbs Buffer.
From www.fishersci.com
Invitrogen PBS PhosphateBuffered Saline (10X) pH 7.4 1LLife Sciences Use Pbs Buffer Phosphate buffers play a critical role in the food services industry in particular. Learn how to better prepare and store your. By preparing 10x instead of 1x pbs solution, you can dilute 100ml of the 10x solution to 900ml of distilled water to obtain 1l of 1x pbs buffer. Phosphate buffered saline (pbs) is a buffer solution commonly used in. Use Pbs Buffer.
From carlova.ee
Intercept® (PBS) Blocking Buffer (500 ml) OÜ Carlova Consult Use Pbs Buffer Learn how to better prepare and store your. By preparing 10x instead of 1x pbs solution, you can dilute 100ml of the 10x solution to 900ml of distilled water to obtain 1l of 1x pbs buffer. Phosphate buffers play a critical role in the food services industry in particular. Buffers and how they control hydrogen ion concentrations, a brief explanation. Use Pbs Buffer.
From alkalisci.com
CellPro™ PBS Buffer ALKALI SCIENTIFIC Use Pbs Buffer The use of a physiological saline buffer is required for different purposes when working with nscs, mainly for tissue or cell. Phosphate buffered saline (pbs) is a buffer solution commonly used in biological research. This technique is commonly used to test certain types of foods. By preparing 10x instead of 1x pbs solution, you can dilute 100ml of the 10x. Use Pbs Buffer.
From 9to5science.com
[Solved] How can I prepare a PBS (Phosphate Buffer 9to5Science Use Pbs Buffer Phosphate buffered saline (pbs) is a buffer solution commonly used in biological research. This technique is commonly used to test certain types of foods. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. Phosphate buffers play a critical role in the food services industry in particular. The. Use Pbs Buffer.
From www.arkanlabs.com
PBS (PhosphateBuffered Saline) Tablets (100 T) Use Pbs Buffer By preparing 10x instead of 1x pbs solution, you can dilute 100ml of the 10x solution to 900ml of distilled water to obtain 1l of 1x pbs buffer. Learn how to better prepare and store your. Phosphate buffered saline (pbs) is a buffer solution commonly used in biological research. Phosphate buffers play a critical role in the food services industry. Use Pbs Buffer.
From www.slideserve.com
PPT Renal Physiology PowerPoint Presentation, free download ID5632772 Use Pbs Buffer Learn how to better prepare and store your. The use of a physiological saline buffer is required for different purposes when working with nscs, mainly for tissue or cell. This technique is commonly used to test certain types of foods. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of. Use Pbs Buffer.
From www.sliderbase.com
Acid and Base Balance and Imbalance Presentation Chemistry Use Pbs Buffer Phosphate buffered saline (pbs) is a buffer solution commonly used in biological research. Learn how to better prepare and store your. By preparing 10x instead of 1x pbs solution, you can dilute 100ml of the 10x solution to 900ml of distilled water to obtain 1l of 1x pbs buffer. Phosphate buffers play a critical role in the food services industry. Use Pbs Buffer.
From sewardjohnsonatelier.org
Custom PBS Buffer Phosphate Buffered Saline (PBS), 53 OFF Use Pbs Buffer The use of a physiological saline buffer is required for different purposes when working with nscs, mainly for tissue or cell. Phosphate buffered saline (pbs) is a buffer solution commonly used in biological research. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. Learn how to better. Use Pbs Buffer.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Use Pbs Buffer This technique is commonly used to test certain types of foods. The use of a physiological saline buffer is required for different purposes when working with nscs, mainly for tissue or cell. Phosphate buffers play a critical role in the food services industry in particular. By preparing 10x instead of 1x pbs solution, you can dilute 100ml of the 10x. Use Pbs Buffer.
From ecatalog.corning.com
Corning® PBS ( Phosphate Buffered Saline) Corning Use Pbs Buffer By preparing 10x instead of 1x pbs solution, you can dilute 100ml of the 10x solution to 900ml of distilled water to obtain 1l of 1x pbs buffer. This technique is commonly used to test certain types of foods. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak. Use Pbs Buffer.
From www.bio-world.com
PBS Buffer 10X, pH 7.4, Sterile bioWORLD Use Pbs Buffer Phosphate buffers play a critical role in the food services industry in particular. This technique is commonly used to test certain types of foods. By preparing 10x instead of 1x pbs solution, you can dilute 100ml of the 10x solution to 900ml of distilled water to obtain 1l of 1x pbs buffer. The use of a physiological saline buffer is. Use Pbs Buffer.
From www.fishersci.fr
Gibco™ PBS (Phosphate Buffered Saline) 10X, pH 7.4 500mL Gibco™ PBS Use Pbs Buffer The use of a physiological saline buffer is required for different purposes when working with nscs, mainly for tissue or cell. Learn how to better prepare and store your. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. Phosphate buffered saline (pbs) is a buffer solution commonly. Use Pbs Buffer.
From www.chegg.com
Solved Phosphate Buffered Saline (PBS) is the most commonly Use Pbs Buffer Learn how to better prepare and store your. Phosphate buffers play a critical role in the food services industry in particular. By preparing 10x instead of 1x pbs solution, you can dilute 100ml of the 10x solution to 900ml of distilled water to obtain 1l of 1x pbs buffer. This technique is commonly used to test certain types of foods.. Use Pbs Buffer.
From www.gbiosciences.com
10X PBS & ReadyToUse 1X PBS (Phosphate Buffered Saline), Sterile Use Pbs Buffer Phosphate buffers play a critical role in the food services industry in particular. The use of a physiological saline buffer is required for different purposes when working with nscs, mainly for tissue or cell. Learn how to better prepare and store your. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium. Use Pbs Buffer.
From www.gbiosciences.com
10X PBS & ReadyToUse 1X PBS (Phosphate Buffered Saline), Sterile Use Pbs Buffer Phosphate buffers play a critical role in the food services industry in particular. By preparing 10x instead of 1x pbs solution, you can dilute 100ml of the 10x solution to 900ml of distilled water to obtain 1l of 1x pbs buffer. This technique is commonly used to test certain types of foods. Phosphate buffered saline (pbs) is a buffer solution. Use Pbs Buffer.
From samatashkhis.com
PBS BUFFER شرکت سما تشخیص آریا Use Pbs Buffer This technique is commonly used to test certain types of foods. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. Learn how to better prepare and store your. Phosphate buffered saline (pbs) is a buffer solution commonly used in biological research. Phosphate buffers play a critical role. Use Pbs Buffer.
From biologixusa.com
Phosphate buffered saline (PBS) Biologix Use Pbs Buffer Learn how to better prepare and store your. Phosphate buffers play a critical role in the food services industry in particular. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. This technique is commonly used to test certain types of foods. By preparing 10x instead of 1x. Use Pbs Buffer.
From www.chegg.com
Solved Phosphate Buffered Saline (PBS) is the most commonly Use Pbs Buffer Phosphate buffered saline (pbs) is a buffer solution commonly used in biological research. Phosphate buffers play a critical role in the food services industry in particular. By preparing 10x instead of 1x pbs solution, you can dilute 100ml of the 10x solution to 900ml of distilled water to obtain 1l of 1x pbs buffer. Learn how to better prepare and. Use Pbs Buffer.
From www.fishersci.ca
MP Biomedicals™ Phosphate Buffered Saline (PBS) Tablets Fisher Scientific Use Pbs Buffer Learn how to better prepare and store your. Phosphate buffered saline (pbs) is a buffer solution commonly used in biological research. The use of a physiological saline buffer is required for different purposes when working with nscs, mainly for tissue or cell. Phosphate buffers play a critical role in the food services industry in particular. This technique is commonly used. Use Pbs Buffer.
From bestonlinecollegesdegrees.com
Pbs Buffer Recipe Besto Blog Use Pbs Buffer Learn how to better prepare and store your. The use of a physiological saline buffer is required for different purposes when working with nscs, mainly for tissue or cell. Phosphate buffers play a critical role in the food services industry in particular. Phosphate buffered saline (pbs) is a buffer solution commonly used in biological research. By preparing 10x instead of. Use Pbs Buffer.
From www.rpicorp.com
P32080500T PBS [Phosphate Buffered Saline] 100 ml Tablets, 500 Use Pbs Buffer Phosphate buffered saline (pbs) is a buffer solution commonly used in biological research. Phosphate buffers play a critical role in the food services industry in particular. This technique is commonly used to test certain types of foods. The use of a physiological saline buffer is required for different purposes when working with nscs, mainly for tissue or cell. By preparing. Use Pbs Buffer.