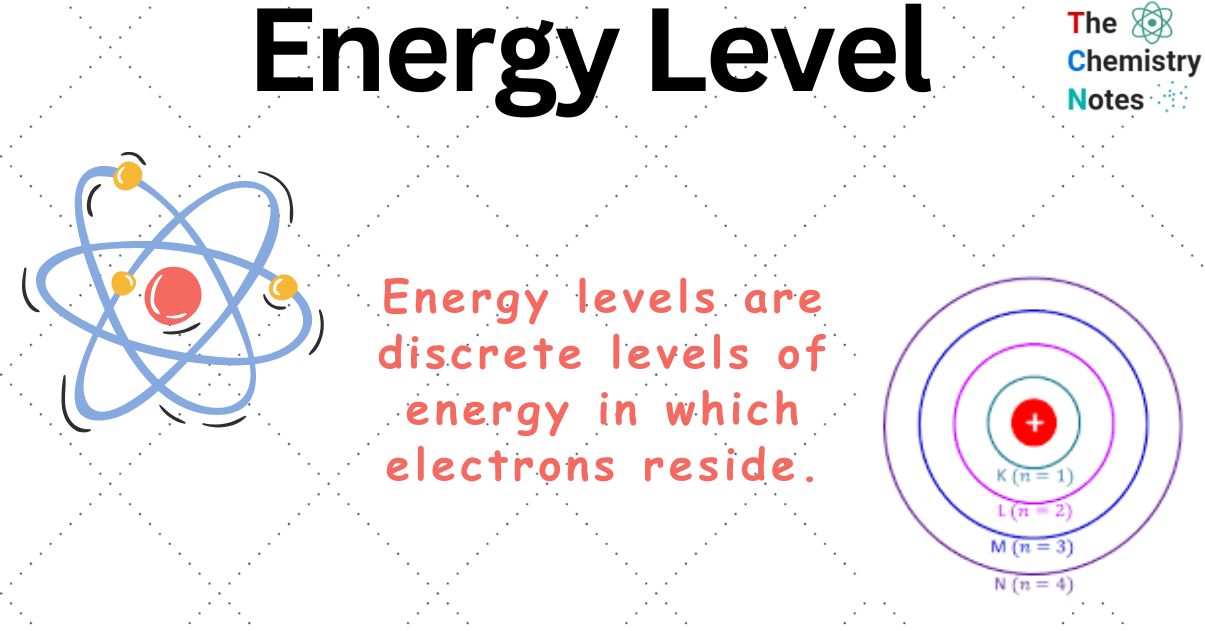
Level Atomic Definition . because the number of protons remains unchanged when an atom forms an ion, the atomic number of the element must be 13. Using these, the quantity of each. According to dalton’s theory atom is smallest particle which could not be divided any further. — chemistry is based on the modern atomic theory, which states that all matter is composed of atoms. atomic number is represented using z and is equal to the number of protons in an atom. — in chemistry, the principal energy level of an electron refers to the shell or orbital in which the electron is located relative to the atom's nucleus. — the laws of quantum mechanics describe the process by which electrons can move from one allowed orbit, or energy level, to another. — energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found.
from thechemistrynotes.com
— energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. — the laws of quantum mechanics describe the process by which electrons can move from one allowed orbit, or energy level, to another. — in chemistry, the principal energy level of an electron refers to the shell or orbital in which the electron is located relative to the atom's nucleus. — chemistry is based on the modern atomic theory, which states that all matter is composed of atoms. Using these, the quantity of each. atomic number is represented using z and is equal to the number of protons in an atom. because the number of protons remains unchanged when an atom forms an ion, the atomic number of the element must be 13. According to dalton’s theory atom is smallest particle which could not be divided any further.
Energy Level, Shell, Subshell Atomic Model
Level Atomic Definition According to dalton’s theory atom is smallest particle which could not be divided any further. — chemistry is based on the modern atomic theory, which states that all matter is composed of atoms. — energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. Using these, the quantity of each. According to dalton’s theory atom is smallest particle which could not be divided any further. — the laws of quantum mechanics describe the process by which electrons can move from one allowed orbit, or energy level, to another. — in chemistry, the principal energy level of an electron refers to the shell or orbital in which the electron is located relative to the atom's nucleus. atomic number is represented using z and is equal to the number of protons in an atom. because the number of protons remains unchanged when an atom forms an ion, the atomic number of the element must be 13.
From study.com
What is an Atom? Lesson for Kids Lesson Level Atomic Definition — in chemistry, the principal energy level of an electron refers to the shell or orbital in which the electron is located relative to the atom's nucleus. Using these, the quantity of each. — chemistry is based on the modern atomic theory, which states that all matter is composed of atoms. atomic number is represented using z. Level Atomic Definition.
From surfguppy.com
Valence Electrons Definition, Obits and Energy Level Level Atomic Definition — chemistry is based on the modern atomic theory, which states that all matter is composed of atoms. — the laws of quantum mechanics describe the process by which electrons can move from one allowed orbit, or energy level, to another. — energy levels (also called electron shells) are fixed distances from the nucleus of an atom. Level Atomic Definition.
From www.sciencefacts.net
Atom Definition, Structure & Parts with Labeled Diagram Level Atomic Definition — energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. — chemistry is based on the modern atomic theory, which states that all matter is composed of atoms. because the number of protons remains unchanged when an atom forms an ion, the atomic number of the. Level Atomic Definition.
From www.online-sciences.com
Atomic structure of matter, Energy levels, Electronic distribution and chemical activity Level Atomic Definition — the laws of quantum mechanics describe the process by which electrons can move from one allowed orbit, or energy level, to another. According to dalton’s theory atom is smallest particle which could not be divided any further. — energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be. Level Atomic Definition.
From www.tes.com
Relative Atomic Mass OCR A Level Teaching Resources Level Atomic Definition Using these, the quantity of each. — the laws of quantum mechanics describe the process by which electrons can move from one allowed orbit, or energy level, to another. — in chemistry, the principal energy level of an electron refers to the shell or orbital in which the electron is located relative to the atom's nucleus. According to. Level Atomic Definition.
From quizlet.com
Diagram and label electron energy levels in Bohr's model. Quizlet Level Atomic Definition — energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. — the laws of quantum mechanics describe the process by which electrons can move from one allowed orbit, or energy level, to another. — chemistry is based on the modern atomic theory, which states that all. Level Atomic Definition.
From www.chemicals.co.uk
A Level Chemistry Revision Physical Chemistry Atomic Structure Level Atomic Definition According to dalton’s theory atom is smallest particle which could not be divided any further. — in chemistry, the principal energy level of an electron refers to the shell or orbital in which the electron is located relative to the atom's nucleus. — the laws of quantum mechanics describe the process by which electrons can move from one. Level Atomic Definition.
From leverageedu.com
Structure Of An Atom Class 9 Science Notes Leverage Edu Level Atomic Definition — chemistry is based on the modern atomic theory, which states that all matter is composed of atoms. According to dalton’s theory atom is smallest particle which could not be divided any further. — energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. — in chemistry,. Level Atomic Definition.
From education-portal.com
What is an Energy Level of an Atom? Definition & Equation Video & Lesson Transcript Level Atomic Definition atomic number is represented using z and is equal to the number of protons in an atom. — in chemistry, the principal energy level of an electron refers to the shell or orbital in which the electron is located relative to the atom's nucleus. According to dalton’s theory atom is smallest particle which could not be divided any. Level Atomic Definition.
From www.britannica.com
Atom Electrons, Nucleus, Bonds Britannica Level Atomic Definition — energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. According to dalton’s theory atom is smallest particle which could not be divided any further. because the number of protons remains unchanged when an atom forms an ion, the atomic number of the element must be 13.. Level Atomic Definition.
From www.britannica.com
Electron Definition, Mass, & Facts Britannica Level Atomic Definition because the number of protons remains unchanged when an atom forms an ion, the atomic number of the element must be 13. — chemistry is based on the modern atomic theory, which states that all matter is composed of atoms. — energy levels (also called electron shells) are fixed distances from the nucleus of an atom where. Level Atomic Definition.
From www.youtube.com
Orbitals, Atomic Energy Levels, & Sublevels Explained Basic Introduction to Quantum Numbers Level Atomic Definition — chemistry is based on the modern atomic theory, which states that all matter is composed of atoms. — energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. — in chemistry, the principal energy level of an electron refers to the shell or orbital in which. Level Atomic Definition.
From spmscience.blog.onlinetuition.com.my
4.2 Structure of Atoms SPM Science Level Atomic Definition — the laws of quantum mechanics describe the process by which electrons can move from one allowed orbit, or energy level, to another. atomic number is represented using z and is equal to the number of protons in an atom. According to dalton’s theory atom is smallest particle which could not be divided any further. — in. Level Atomic Definition.
From wghsjuniorscience.weebly.com
Atomic structure WGHS Junior Science Level Atomic Definition — in chemistry, the principal energy level of an electron refers to the shell or orbital in which the electron is located relative to the atom's nucleus. — chemistry is based on the modern atomic theory, which states that all matter is composed of atoms. because the number of protons remains unchanged when an atom forms an. Level Atomic Definition.
From circuitpennhockey01yh.z13.web.core.windows.net
Atom With A Charge Level Atomic Definition — the laws of quantum mechanics describe the process by which electrons can move from one allowed orbit, or energy level, to another. Using these, the quantity of each. — chemistry is based on the modern atomic theory, which states that all matter is composed of atoms. According to dalton’s theory atom is smallest particle which could not. Level Atomic Definition.
From hubpages.com
Atoms and Atomic Structure HubPages Level Atomic Definition Using these, the quantity of each. because the number of protons remains unchanged when an atom forms an ion, the atomic number of the element must be 13. — energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. — chemistry is based on the modern atomic. Level Atomic Definition.
From www.biologyonline.com
Atom Definition and Examples Biology Online Dictionary Level Atomic Definition According to dalton’s theory atom is smallest particle which could not be divided any further. — the laws of quantum mechanics describe the process by which electrons can move from one allowed orbit, or energy level, to another. atomic number is represented using z and is equal to the number of protons in an atom. — energy. Level Atomic Definition.
From pijaeducation.com
ATOM, ORBITS AND ENERGY LEVELS » PIJA Education Level Atomic Definition According to dalton’s theory atom is smallest particle which could not be divided any further. — in chemistry, the principal energy level of an electron refers to the shell or orbital in which the electron is located relative to the atom's nucleus. because the number of protons remains unchanged when an atom forms an ion, the atomic number. Level Atomic Definition.
From twinklsecondary.blog
A Level Atomic Structure A Level Chemistry Revision Level Atomic Definition atomic number is represented using z and is equal to the number of protons in an atom. — in chemistry, the principal energy level of an electron refers to the shell or orbital in which the electron is located relative to the atom's nucleus. According to dalton’s theory atom is smallest particle which could not be divided any. Level Atomic Definition.
From www.slideserve.com
PPT ATOMIC THEORY REVIEW PowerPoint Presentation, free download ID5479628 Level Atomic Definition — in chemistry, the principal energy level of an electron refers to the shell or orbital in which the electron is located relative to the atom's nucleus. — chemistry is based on the modern atomic theory, which states that all matter is composed of atoms. Using these, the quantity of each. — the laws of quantum mechanics. Level Atomic Definition.
From mungfali.com
Label The Parts Of An Atom Level Atomic Definition — chemistry is based on the modern atomic theory, which states that all matter is composed of atoms. — in chemistry, the principal energy level of an electron refers to the shell or orbital in which the electron is located relative to the atom's nucleus. — energy levels (also called electron shells) are fixed distances from the. Level Atomic Definition.
From www.smartexamresources.com
IGCSE Chemistry Notes Atomic Structure And The Periodic Table Smart Exam Resources Level Atomic Definition According to dalton’s theory atom is smallest particle which could not be divided any further. — chemistry is based on the modern atomic theory, which states that all matter is composed of atoms. — energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. Using these, the quantity. Level Atomic Definition.
From www.broadlearnings.com
Atomic Structure Broad Learnings Level Atomic Definition — chemistry is based on the modern atomic theory, which states that all matter is composed of atoms. atomic number is represented using z and is equal to the number of protons in an atom. Using these, the quantity of each. — in chemistry, the principal energy level of an electron refers to the shell or orbital. Level Atomic Definition.
From study.com
Atom Definition, Structure & Examples Lesson Level Atomic Definition atomic number is represented using z and is equal to the number of protons in an atom. According to dalton’s theory atom is smallest particle which could not be divided any further. — the laws of quantum mechanics describe the process by which electrons can move from one allowed orbit, or energy level, to another. because the. Level Atomic Definition.
From learningschoolequalrf.z22.web.core.windows.net
Explain Bohr's Atomic Model Level Atomic Definition According to dalton’s theory atom is smallest particle which could not be divided any further. — chemistry is based on the modern atomic theory, which states that all matter is composed of atoms. because the number of protons remains unchanged when an atom forms an ion, the atomic number of the element must be 13. atomic number. Level Atomic Definition.
From acemichael888.weebly.com
Electron Arrangement in Atoms Elements and the Periodic Table Level Atomic Definition Using these, the quantity of each. because the number of protons remains unchanged when an atom forms an ion, the atomic number of the element must be 13. — energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. atomic number is represented using z and is. Level Atomic Definition.
From sites.google.com
Structure of the Atom Unit G Review Level Atomic Definition — chemistry is based on the modern atomic theory, which states that all matter is composed of atoms. — in chemistry, the principal energy level of an electron refers to the shell or orbital in which the electron is located relative to the atom's nucleus. because the number of protons remains unchanged when an atom forms an. Level Atomic Definition.
From www.carlsonstockart.com
Electron Energy Levels of Atoms Image License Carlson Stock Art Level Atomic Definition atomic number is represented using z and is equal to the number of protons in an atom. — the laws of quantum mechanics describe the process by which electrons can move from one allowed orbit, or energy level, to another. — chemistry is based on the modern atomic theory, which states that all matter is composed of. Level Atomic Definition.
From www.chemicals.co.uk
Chemistry GCSE Revision Atomic Structure And The Periodic Table Level Atomic Definition — chemistry is based on the modern atomic theory, which states that all matter is composed of atoms. According to dalton’s theory atom is smallest particle which could not be divided any further. — the laws of quantum mechanics describe the process by which electrons can move from one allowed orbit, or energy level, to another. atomic. Level Atomic Definition.
From www.worksheetsplanet.com
What is an Atom Meaning & Definition of Atom Level Atomic Definition because the number of protons remains unchanged when an atom forms an ion, the atomic number of the element must be 13. — energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. atomic number is represented using z and is equal to the number of protons. Level Atomic Definition.
From www.slideserve.com
PPT Atomic Structure and Atomic Spectra (CH 13) PowerPoint Presentation ID511966 Level Atomic Definition atomic number is represented using z and is equal to the number of protons in an atom. — in chemistry, the principal energy level of an electron refers to the shell or orbital in which the electron is located relative to the atom's nucleus. — the laws of quantum mechanics describe the process by which electrons can. Level Atomic Definition.
From www.slideserve.com
PPT Chapters 45 The Atom PowerPoint Presentation, free download ID179228 Level Atomic Definition — energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. because the number of protons remains unchanged when an atom forms an ion, the atomic number of the element must be 13. According to dalton’s theory atom is smallest particle which could not be divided any further.. Level Atomic Definition.
From hubpages.com
Atoms and Atomic Structure Level Atomic Definition — energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. because the number of protons remains unchanged when an atom forms an ion, the atomic number of the element must be 13. — in chemistry, the principal energy level of an electron refers to the shell. Level Atomic Definition.
From thechemistrynotes.com
Energy Level, Shell, Subshell Atomic Model Level Atomic Definition — chemistry is based on the modern atomic theory, which states that all matter is composed of atoms. — the laws of quantum mechanics describe the process by which electrons can move from one allowed orbit, or energy level, to another. because the number of protons remains unchanged when an atom forms an ion, the atomic number. Level Atomic Definition.
From www.youtube.com
Chemistry Basics Atom Structure, Energy Levels, Sublevels, Orbitals YouTube Level Atomic Definition — chemistry is based on the modern atomic theory, which states that all matter is composed of atoms. atomic number is represented using z and is equal to the number of protons in an atom. — in chemistry, the principal energy level of an electron refers to the shell or orbital in which the electron is located. Level Atomic Definition.