What Is The Relationship Between Ph And Concentration . In general, the ph of an acid solution will be lower as the solution is more concentrated. For bases, the ph will be higher with more highly. The ph value is an. Ph and poh are defined as the negative log of hydrogen ion concentration and hydroxide concentration, respectively. For example, at a ph of zero the hydronium ion concentration is one molar, while at ph 14 the hydroxide ion concentration is one molar. Pka (acid dissociation constant) and ph are related, but pka is more specific in that it helps you predict what a. It states that ph equals the negative logarithmic value of hydrogen ion (h +) concentration. Poh is related to ph and can be easily calculated from. With this ph calculator, you can determine the ph of a solution in a few ways. It can convert ph to h +, as well as calculate ph from the ionization constant and concentration. The ph of a solution is a measure of the concentration of hydrogen ions.
from guides.hostos.cuny.edu
Ph and poh are defined as the negative log of hydrogen ion concentration and hydroxide concentration, respectively. The ph value is an. For example, at a ph of zero the hydronium ion concentration is one molar, while at ph 14 the hydroxide ion concentration is one molar. The ph of a solution is a measure of the concentration of hydrogen ions. Poh is related to ph and can be easily calculated from. In general, the ph of an acid solution will be lower as the solution is more concentrated. Pka (acid dissociation constant) and ph are related, but pka is more specific in that it helps you predict what a. For bases, the ph will be higher with more highly. It can convert ph to h +, as well as calculate ph from the ionization constant and concentration. With this ph calculator, you can determine the ph of a solution in a few ways.
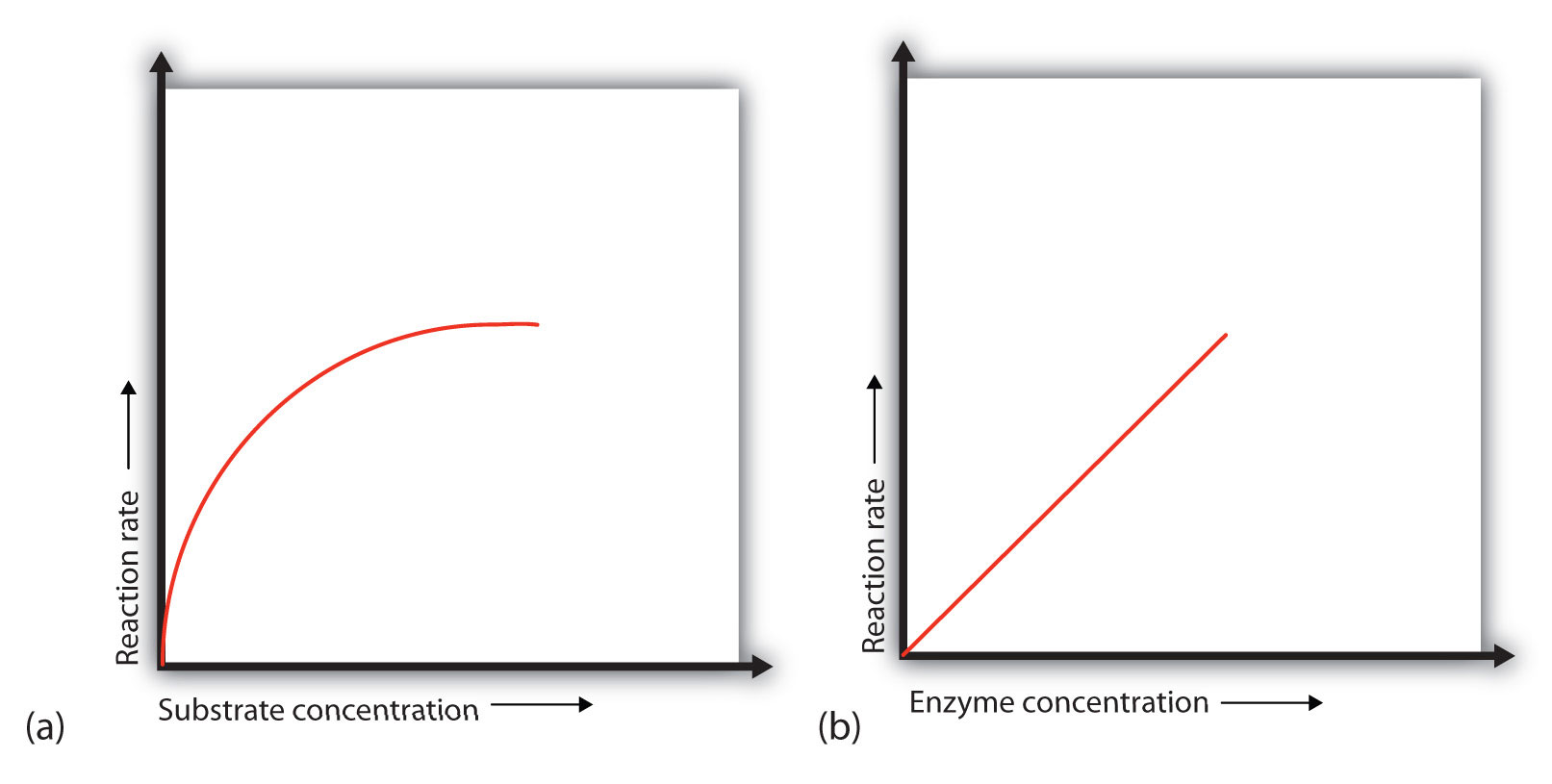
Chapter 9 Proteins and Enzymes CHE 120 Introduction to Organic
What Is The Relationship Between Ph And Concentration With this ph calculator, you can determine the ph of a solution in a few ways. With this ph calculator, you can determine the ph of a solution in a few ways. Poh is related to ph and can be easily calculated from. For bases, the ph will be higher with more highly. In general, the ph of an acid solution will be lower as the solution is more concentrated. It states that ph equals the negative logarithmic value of hydrogen ion (h +) concentration. It can convert ph to h +, as well as calculate ph from the ionization constant and concentration. The ph value is an. Pka (acid dissociation constant) and ph are related, but pka is more specific in that it helps you predict what a. Ph and poh are defined as the negative log of hydrogen ion concentration and hydroxide concentration, respectively. The ph of a solution is a measure of the concentration of hydrogen ions. For example, at a ph of zero the hydronium ion concentration is one molar, while at ph 14 the hydroxide ion concentration is one molar.
From www.researchgate.net
The measured pH values of different sodium carbonate and sodium What Is The Relationship Between Ph And Concentration It states that ph equals the negative logarithmic value of hydrogen ion (h +) concentration. For example, at a ph of zero the hydronium ion concentration is one molar, while at ph 14 the hydroxide ion concentration is one molar. It can convert ph to h +, as well as calculate ph from the ionization constant and concentration. Pka (acid. What Is The Relationship Between Ph And Concentration.
From www.researchgate.net
Relationship between pH and concentration of P adsorbed onto (a What Is The Relationship Between Ph And Concentration With this ph calculator, you can determine the ph of a solution in a few ways. In general, the ph of an acid solution will be lower as the solution is more concentrated. It states that ph equals the negative logarithmic value of hydrogen ion (h +) concentration. The ph of a solution is a measure of the concentration of. What Is The Relationship Between Ph And Concentration.
From www.chegg.com
Solved When comparing solutions of equal concentration, what What Is The Relationship Between Ph And Concentration Ph and poh are defined as the negative log of hydrogen ion concentration and hydroxide concentration, respectively. In general, the ph of an acid solution will be lower as the solution is more concentrated. It can convert ph to h +, as well as calculate ph from the ionization constant and concentration. The ph value is an. With this ph. What Is The Relationship Between Ph And Concentration.
From www.researchgate.net
Relationship between pH and the concentration of the dissolved heavy What Is The Relationship Between Ph And Concentration For bases, the ph will be higher with more highly. With this ph calculator, you can determine the ph of a solution in a few ways. For example, at a ph of zero the hydronium ion concentration is one molar, while at ph 14 the hydroxide ion concentration is one molar. Ph and poh are defined as the negative log. What Is The Relationship Between Ph And Concentration.
From gbu-taganskij.ru
PH POH LabXchange, 54 OFF gbutaganskij.ru What Is The Relationship Between Ph And Concentration The ph value is an. Ph and poh are defined as the negative log of hydrogen ion concentration and hydroxide concentration, respectively. It states that ph equals the negative logarithmic value of hydrogen ion (h +) concentration. Pka (acid dissociation constant) and ph are related, but pka is more specific in that it helps you predict what a. In general,. What Is The Relationship Between Ph And Concentration.
From worldnewlive.com
When PKa Is Equal To PH? Mastery Wiki What Is The Relationship Between Ph And Concentration Ph and poh are defined as the negative log of hydrogen ion concentration and hydroxide concentration, respectively. For example, at a ph of zero the hydronium ion concentration is one molar, while at ph 14 the hydroxide ion concentration is one molar. It states that ph equals the negative logarithmic value of hydrogen ion (h +) concentration. With this ph. What Is The Relationship Between Ph And Concentration.
From techblog.ctgclean.com
Chemistry The "Trouble" With pH CTG Clean What Is The Relationship Between Ph And Concentration It can convert ph to h +, as well as calculate ph from the ionization constant and concentration. Pka (acid dissociation constant) and ph are related, but pka is more specific in that it helps you predict what a. In general, the ph of an acid solution will be lower as the solution is more concentrated. It states that ph. What Is The Relationship Between Ph And Concentration.
From www.researchgate.net
The relationship between pH and concentration compared to the results What Is The Relationship Between Ph And Concentration The ph of a solution is a measure of the concentration of hydrogen ions. Ph and poh are defined as the negative log of hydrogen ion concentration and hydroxide concentration, respectively. Poh is related to ph and can be easily calculated from. In general, the ph of an acid solution will be lower as the solution is more concentrated. It. What Is The Relationship Between Ph And Concentration.
From www.researchgate.net
Relationship between NaOH dosage and pH. Conditions NaOH What Is The Relationship Between Ph And Concentration For bases, the ph will be higher with more highly. Ph and poh are defined as the negative log of hydrogen ion concentration and hydroxide concentration, respectively. The ph value is an. In general, the ph of an acid solution will be lower as the solution is more concentrated. With this ph calculator, you can determine the ph of a. What Is The Relationship Between Ph And Concentration.
From www.bartleby.com
The following graph shows the relationship between enzyme activity and What Is The Relationship Between Ph And Concentration The ph value is an. Ph and poh are defined as the negative log of hydrogen ion concentration and hydroxide concentration, respectively. In general, the ph of an acid solution will be lower as the solution is more concentrated. Pka (acid dissociation constant) and ph are related, but pka is more specific in that it helps you predict what a.. What Is The Relationship Between Ph And Concentration.
From www.researchgate.net
The relationship between pH and concentration compared to the results What Is The Relationship Between Ph And Concentration Poh is related to ph and can be easily calculated from. In general, the ph of an acid solution will be lower as the solution is more concentrated. It states that ph equals the negative logarithmic value of hydrogen ion (h +) concentration. For example, at a ph of zero the hydronium ion concentration is one molar, while at ph. What Is The Relationship Between Ph And Concentration.
From www.shutterstock.com
Ph Scale Chart Corresponding Hydrogen Ion Stock Vector 293946068 What Is The Relationship Between Ph And Concentration In general, the ph of an acid solution will be lower as the solution is more concentrated. For example, at a ph of zero the hydronium ion concentration is one molar, while at ph 14 the hydroxide ion concentration is one molar. It can convert ph to h +, as well as calculate ph from the ionization constant and concentration.. What Is The Relationship Between Ph And Concentration.
From brainly.in
Observe the graph drawn between concentration of H3 O^+ Vs pH. Observe What Is The Relationship Between Ph And Concentration In general, the ph of an acid solution will be lower as the solution is more concentrated. Ph and poh are defined as the negative log of hydrogen ion concentration and hydroxide concentration, respectively. The ph value is an. With this ph calculator, you can determine the ph of a solution in a few ways. It can convert ph to. What Is The Relationship Between Ph And Concentration.
From guides.hostos.cuny.edu
Chapter 9 Proteins and Enzymes CHE 120 Introduction to Organic What Is The Relationship Between Ph And Concentration Ph and poh are defined as the negative log of hydrogen ion concentration and hydroxide concentration, respectively. The ph value is an. Poh is related to ph and can be easily calculated from. It states that ph equals the negative logarithmic value of hydrogen ion (h +) concentration. With this ph calculator, you can determine the ph of a solution. What Is The Relationship Between Ph And Concentration.
From www.youtube.com
pH Hydrogen Ion Concentration Normal pH Range Survival Range What Is The Relationship Between Ph And Concentration Poh is related to ph and can be easily calculated from. In general, the ph of an acid solution will be lower as the solution is more concentrated. For example, at a ph of zero the hydronium ion concentration is one molar, while at ph 14 the hydroxide ion concentration is one molar. For bases, the ph will be higher. What Is The Relationship Between Ph And Concentration.
From saylordotorg.github.io
Enzyme Activity What Is The Relationship Between Ph And Concentration It can convert ph to h +, as well as calculate ph from the ionization constant and concentration. It states that ph equals the negative logarithmic value of hydrogen ion (h +) concentration. The ph of a solution is a measure of the concentration of hydrogen ions. Pka (acid dissociation constant) and ph are related, but pka is more specific. What Is The Relationship Between Ph And Concentration.
From pediaa.com
Relationship Between Hydrogen Ions and pH Definition, Properties, pH What Is The Relationship Between Ph And Concentration In general, the ph of an acid solution will be lower as the solution is more concentrated. Ph and poh are defined as the negative log of hydrogen ion concentration and hydroxide concentration, respectively. Pka (acid dissociation constant) and ph are related, but pka is more specific in that it helps you predict what a. Poh is related to ph. What Is The Relationship Between Ph And Concentration.
From www.nagwa.com
Question Video Calculating the pH of a Solution from the Hydroxide Ion What Is The Relationship Between Ph And Concentration It can convert ph to h +, as well as calculate ph from the ionization constant and concentration. With this ph calculator, you can determine the ph of a solution in a few ways. The ph of a solution is a measure of the concentration of hydrogen ions. In general, the ph of an acid solution will be lower as. What Is The Relationship Between Ph And Concentration.
From www.slideserve.com
PPT Acids and Bases 9 / 03 / 2009 PowerPoint Presentation, free What Is The Relationship Between Ph And Concentration The ph value is an. In general, the ph of an acid solution will be lower as the solution is more concentrated. For example, at a ph of zero the hydronium ion concentration is one molar, while at ph 14 the hydroxide ion concentration is one molar. Poh is related to ph and can be easily calculated from. For bases,. What Is The Relationship Between Ph And Concentration.
From www.ukessays.com
Relationship Between the Concentration of Atmospheric Carbon Dioxide What Is The Relationship Between Ph And Concentration With this ph calculator, you can determine the ph of a solution in a few ways. Pka (acid dissociation constant) and ph are related, but pka is more specific in that it helps you predict what a. It can convert ph to h +, as well as calculate ph from the ionization constant and concentration. The ph value is an.. What Is The Relationship Between Ph And Concentration.
From www.youtube.com
Hydrogen Ion and Hydroxide Ion Concentrations (Example) YouTube What Is The Relationship Between Ph And Concentration It states that ph equals the negative logarithmic value of hydrogen ion (h +) concentration. Poh is related to ph and can be easily calculated from. With this ph calculator, you can determine the ph of a solution in a few ways. The ph value is an. It can convert ph to h +, as well as calculate ph from. What Is The Relationship Between Ph And Concentration.
From www.chegg.com
Solved The following plots illustrate the effect of pH, What Is The Relationship Between Ph And Concentration In general, the ph of an acid solution will be lower as the solution is more concentrated. Ph and poh are defined as the negative log of hydrogen ion concentration and hydroxide concentration, respectively. Pka (acid dissociation constant) and ph are related, but pka is more specific in that it helps you predict what a. It can convert ph to. What Is The Relationship Between Ph And Concentration.
From www.av8n.com
pH versus Concentration What Is The Relationship Between Ph And Concentration Poh is related to ph and can be easily calculated from. Pka (acid dissociation constant) and ph are related, but pka is more specific in that it helps you predict what a. In general, the ph of an acid solution will be lower as the solution is more concentrated. For example, at a ph of zero the hydronium ion concentration. What Is The Relationship Between Ph And Concentration.
From blog.nus.edu.sg
pH and environmental pollution Environmental Pollution What Is The Relationship Between Ph And Concentration Poh is related to ph and can be easily calculated from. Pka (acid dissociation constant) and ph are related, but pka is more specific in that it helps you predict what a. Ph and poh are defined as the negative log of hydrogen ion concentration and hydroxide concentration, respectively. The ph value is an. It can convert ph to h. What Is The Relationship Between Ph And Concentration.
From www.compoundchem.com
A Guide to Acids, Acid Strength, and Concentration Compound Interest What Is The Relationship Between Ph And Concentration For example, at a ph of zero the hydronium ion concentration is one molar, while at ph 14 the hydroxide ion concentration is one molar. The ph of a solution is a measure of the concentration of hydrogen ions. Poh is related to ph and can be easily calculated from. Pka (acid dissociation constant) and ph are related, but pka. What Is The Relationship Between Ph And Concentration.
From www.undergraceovercoffee.com
Caustic Soda Concentration Vs Ph Chart Reviews Of Chart What Is The Relationship Between Ph And Concentration The ph of a solution is a measure of the concentration of hydrogen ions. It can convert ph to h +, as well as calculate ph from the ionization constant and concentration. For example, at a ph of zero the hydronium ion concentration is one molar, while at ph 14 the hydroxide ion concentration is one molar. Poh is related. What Is The Relationship Between Ph And Concentration.
From shaunmwilliams.com
Chapter 13 Presentation What Is The Relationship Between Ph And Concentration For example, at a ph of zero the hydronium ion concentration is one molar, while at ph 14 the hydroxide ion concentration is one molar. It can convert ph to h +, as well as calculate ph from the ionization constant and concentration. It states that ph equals the negative logarithmic value of hydrogen ion (h +) concentration. In general,. What Is The Relationship Between Ph And Concentration.
From fr.thptnganamst.edu.vn
Mise à jour 50+ imagen formule ph concentration fr.thptnganamst.edu.vn What Is The Relationship Between Ph And Concentration In general, the ph of an acid solution will be lower as the solution is more concentrated. It states that ph equals the negative logarithmic value of hydrogen ion (h +) concentration. The ph of a solution is a measure of the concentration of hydrogen ions. With this ph calculator, you can determine the ph of a solution in a. What Is The Relationship Between Ph And Concentration.
From courses.lumenlearning.com
14.2 pH and pOH Chemistry What Is The Relationship Between Ph And Concentration In general, the ph of an acid solution will be lower as the solution is more concentrated. Pka (acid dissociation constant) and ph are related, but pka is more specific in that it helps you predict what a. Ph and poh are defined as the negative log of hydrogen ion concentration and hydroxide concentration, respectively. It can convert ph to. What Is The Relationship Between Ph And Concentration.
From schoolworkhelper.net
Effect of Temperature, pH, and Substrate Concentration on Enzyme What Is The Relationship Between Ph And Concentration For example, at a ph of zero the hydronium ion concentration is one molar, while at ph 14 the hydroxide ion concentration is one molar. For bases, the ph will be higher with more highly. Poh is related to ph and can be easily calculated from. In general, the ph of an acid solution will be lower as the solution. What Is The Relationship Between Ph And Concentration.
From www.slideserve.com
PPT BCHS 3304 General Biochemistry I, Section 07553 Spring 2003 100 What Is The Relationship Between Ph And Concentration With this ph calculator, you can determine the ph of a solution in a few ways. It states that ph equals the negative logarithmic value of hydrogen ion (h +) concentration. Pka (acid dissociation constant) and ph are related, but pka is more specific in that it helps you predict what a. For example, at a ph of zero the. What Is The Relationship Between Ph And Concentration.
From preparatorychemistry.com
pH and Equilibrium What Is The Relationship Between Ph And Concentration It can convert ph to h +, as well as calculate ph from the ionization constant and concentration. The ph value is an. In general, the ph of an acid solution will be lower as the solution is more concentrated. The ph of a solution is a measure of the concentration of hydrogen ions. For bases, the ph will be. What Is The Relationship Between Ph And Concentration.
From www.youtube.com
acid base chemistry relation between pH pOH [H] [OH] YouTube What Is The Relationship Between Ph And Concentration The ph of a solution is a measure of the concentration of hydrogen ions. It states that ph equals the negative logarithmic value of hydrogen ion (h +) concentration. For bases, the ph will be higher with more highly. It can convert ph to h +, as well as calculate ph from the ionization constant and concentration. Ph and poh. What Is The Relationship Between Ph And Concentration.
From dylanmeowbutler.blogspot.com
How to Calculate Ph What Is The Relationship Between Ph And Concentration It states that ph equals the negative logarithmic value of hydrogen ion (h +) concentration. The ph value is an. For example, at a ph of zero the hydronium ion concentration is one molar, while at ph 14 the hydroxide ion concentration is one molar. For bases, the ph will be higher with more highly. In general, the ph of. What Is The Relationship Between Ph And Concentration.
From dxokntnej.blob.core.windows.net
Relationship Between Ph And Buffer Capacity at Richard Wise blog What Is The Relationship Between Ph And Concentration With this ph calculator, you can determine the ph of a solution in a few ways. The ph of a solution is a measure of the concentration of hydrogen ions. Ph and poh are defined as the negative log of hydrogen ion concentration and hydroxide concentration, respectively. For bases, the ph will be higher with more highly. Pka (acid dissociation. What Is The Relationship Between Ph And Concentration.