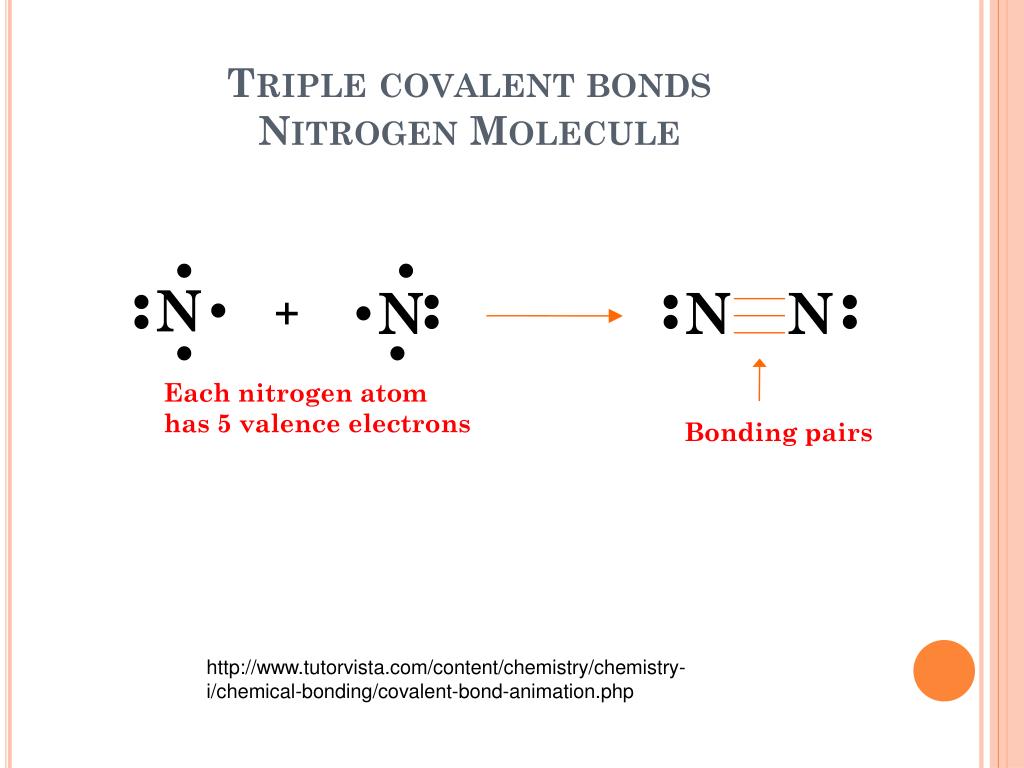
Why Does Nitrogen Have A Lone Pair . Nitrogen has one lone pair of electrons in its 2s orbital. As with so 2, this composite model of electron distribution and negative electrostatic potential in ammonia shows that a lone pair of electrons occupies a larger region of space around the nitrogen atom than does a bonding pair of electrons that is shared with a hydrogen atom. The nitrogen with a double bond forms two σ bonds with two of its sp 2 orbitals and uses the third to accommodate its lone pair. Nitrogen's maximum covalency is indeed $4$. Have a look at the lewis structure of the. And no, it does not break up its lone pair. This coordinate bond that nitrogen forms by donating its. It can donate this electron pair to form a coordinate bond. The other is involved in a pi bond, and. I'll give you a simple example. The lone pair electrons on the nitrogen are contained in the last sp 3 hybridized orbital. These three bonds involve three of nitrogen’s valence electrons; The remaining two valence electrons occupy a non. In ammonia the nitrogen atom is bonded to three hydrogen atoms. Due to the sp 3 hybridization the nitrogen has a.
from www.slideserve.com
And no, it does not break up its lone pair. In ammonia the nitrogen atom is bonded to three hydrogen atoms. The remaining two valence electrons occupy a non. Due to the sp 3 hybridization the nitrogen has a. Have a look at the lewis structure of the. As with so 2, this composite model of electron distribution and negative electrostatic potential in ammonia shows that a lone pair of electrons occupies a larger region of space around the nitrogen atom than does a bonding pair of electrons that is shared with a hydrogen atom. Nitrogen's maximum covalency is indeed $4$. This coordinate bond that nitrogen forms by donating its. The lone pair electrons on the nitrogen are contained in the last sp 3 hybridized orbital. The nitrogen with a double bond forms two σ bonds with two of its sp 2 orbitals and uses the third to accommodate its lone pair.
PPT COVALENT BONDING PowerPoint Presentation, free download ID5128236
Why Does Nitrogen Have A Lone Pair The nitrogen with a double bond forms two σ bonds with two of its sp 2 orbitals and uses the third to accommodate its lone pair. The other is involved in a pi bond, and. The nitrogen with a double bond forms two σ bonds with two of its sp 2 orbitals and uses the third to accommodate its lone pair. In ammonia the nitrogen atom is bonded to three hydrogen atoms. Nitrogen has one lone pair of electrons in its 2s orbital. It can donate this electron pair to form a coordinate bond. Have a look at the lewis structure of the. The lone pair electrons on the nitrogen are contained in the last sp 3 hybridized orbital. These three bonds involve three of nitrogen’s valence electrons; I'll give you a simple example. As with so 2, this composite model of electron distribution and negative electrostatic potential in ammonia shows that a lone pair of electrons occupies a larger region of space around the nitrogen atom than does a bonding pair of electrons that is shared with a hydrogen atom. The remaining two valence electrons occupy a non. And no, it does not break up its lone pair. Nitrogen's maximum covalency is indeed $4$. Due to the sp 3 hybridization the nitrogen has a. This coordinate bond that nitrogen forms by donating its.
From brainly.in
In which of the following lone pair of nitrogen is not involved in Why Does Nitrogen Have A Lone Pair As with so 2, this composite model of electron distribution and negative electrostatic potential in ammonia shows that a lone pair of electrons occupies a larger region of space around the nitrogen atom than does a bonding pair of electrons that is shared with a hydrogen atom. It can donate this electron pair to form a coordinate bond. Have a. Why Does Nitrogen Have A Lone Pair.
From www.numerade.com
Consider the nitrogen atoms (labeled AD) in the molecule of Caffeine Why Does Nitrogen Have A Lone Pair The remaining two valence electrons occupy a non. Nitrogen has one lone pair of electrons in its 2s orbital. These three bonds involve three of nitrogen’s valence electrons; The lone pair electrons on the nitrogen are contained in the last sp 3 hybridized orbital. Have a look at the lewis structure of the. The nitrogen with a double bond forms. Why Does Nitrogen Have A Lone Pair.
From www.youtube.com
Number of Lone Pairs and Bonding Pairs for CO2 (Carbon dioxide) YouTube Why Does Nitrogen Have A Lone Pair The nitrogen with a double bond forms two σ bonds with two of its sp 2 orbitals and uses the third to accommodate its lone pair. As with so 2, this composite model of electron distribution and negative electrostatic potential in ammonia shows that a lone pair of electrons occupies a larger region of space around the nitrogen atom than. Why Does Nitrogen Have A Lone Pair.
From chemistry.stackexchange.com
organic chemistry Why is the lone pair of pyridine's nitrogen atom Why Does Nitrogen Have A Lone Pair These three bonds involve three of nitrogen’s valence electrons; It can donate this electron pair to form a coordinate bond. The remaining two valence electrons occupy a non. In ammonia the nitrogen atom is bonded to three hydrogen atoms. The nitrogen with a double bond forms two σ bonds with two of its sp 2 orbitals and uses the third. Why Does Nitrogen Have A Lone Pair.
From socratic.org
Question 75b36 Socratic Why Does Nitrogen Have A Lone Pair Have a look at the lewis structure of the. The lone pair electrons on the nitrogen are contained in the last sp 3 hybridized orbital. As with so 2, this composite model of electron distribution and negative electrostatic potential in ammonia shows that a lone pair of electrons occupies a larger region of space around the nitrogen atom than does. Why Does Nitrogen Have A Lone Pair.
From www.slideserve.com
PPT COVALENT BONDING PowerPoint Presentation, free download ID5128236 Why Does Nitrogen Have A Lone Pair These three bonds involve three of nitrogen’s valence electrons; It can donate this electron pair to form a coordinate bond. As with so 2, this composite model of electron distribution and negative electrostatic potential in ammonia shows that a lone pair of electrons occupies a larger region of space around the nitrogen atom than does a bonding pair of electrons. Why Does Nitrogen Have A Lone Pair.
From chamotgallery.com
Nitrogen(N) electron configuration and orbital diagram (2022) Why Does Nitrogen Have A Lone Pair I'll give you a simple example. It can donate this electron pair to form a coordinate bond. The nitrogen with a double bond forms two σ bonds with two of its sp 2 orbitals and uses the third to accommodate its lone pair. Nitrogen has one lone pair of electrons in its 2s orbital. The other is involved in a. Why Does Nitrogen Have A Lone Pair.
From www.chegg.com
Solved 12. The lone pair on the indicated nitrogen is in Why Does Nitrogen Have A Lone Pair Due to the sp 3 hybridization the nitrogen has a. The other is involved in a pi bond, and. Nitrogen has one lone pair of electrons in its 2s orbital. It can donate this electron pair to form a coordinate bond. This coordinate bond that nitrogen forms by donating its. And no, it does not break up its lone pair.. Why Does Nitrogen Have A Lone Pair.
From byjus.com
Does Water have 2 Lone Pairs? Why Does Nitrogen Have A Lone Pair The nitrogen with a double bond forms two σ bonds with two of its sp 2 orbitals and uses the third to accommodate its lone pair. Have a look at the lewis structure of the. These three bonds involve three of nitrogen’s valence electrons; This coordinate bond that nitrogen forms by donating its. It can donate this electron pair to. Why Does Nitrogen Have A Lone Pair.
From flatworldknowledge.lardbucket.org
Predicting the Geometry of Molecules and Polyatomic Ions Why Does Nitrogen Have A Lone Pair I'll give you a simple example. And no, it does not break up its lone pair. The lone pair electrons on the nitrogen are contained in the last sp 3 hybridized orbital. The nitrogen with a double bond forms two σ bonds with two of its sp 2 orbitals and uses the third to accommodate its lone pair. The other. Why Does Nitrogen Have A Lone Pair.
From www.slideserve.com
PPT Structure and Bonding PowerPoint Presentation, free download ID Why Does Nitrogen Have A Lone Pair Nitrogen has one lone pair of electrons in its 2s orbital. These three bonds involve three of nitrogen’s valence electrons; In ammonia the nitrogen atom is bonded to three hydrogen atoms. Have a look at the lewis structure of the. The nitrogen with a double bond forms two σ bonds with two of its sp 2 orbitals and uses the. Why Does Nitrogen Have A Lone Pair.
From www.youtube.com
In NO3 ion, number of bond pair and lone pair of electrons on Why Does Nitrogen Have A Lone Pair The other is involved in a pi bond, and. In ammonia the nitrogen atom is bonded to three hydrogen atoms. It can donate this electron pair to form a coordinate bond. Have a look at the lewis structure of the. And no, it does not break up its lone pair. The nitrogen with a double bond forms two σ bonds. Why Does Nitrogen Have A Lone Pair.
From courses.lumenlearning.com
Valence Bond Theory MCC Organic Chemistry Why Does Nitrogen Have A Lone Pair The lone pair electrons on the nitrogen are contained in the last sp 3 hybridized orbital. I'll give you a simple example. And no, it does not break up its lone pair. The other is involved in a pi bond, and. In ammonia the nitrogen atom is bonded to three hydrogen atoms. The remaining two valence electrons occupy a non.. Why Does Nitrogen Have A Lone Pair.
From www.youtube.com
Formal Charge Key Patterns for Carbon, Nitrogen, Oxygen YouTube Why Does Nitrogen Have A Lone Pair These three bonds involve three of nitrogen’s valence electrons; Nitrogen's maximum covalency is indeed $4$. The remaining two valence electrons occupy a non. The other is involved in a pi bond, and. This coordinate bond that nitrogen forms by donating its. I'll give you a simple example. The lone pair electrons on the nitrogen are contained in the last sp. Why Does Nitrogen Have A Lone Pair.
From ask4essay.com
How many valence electrons does nitrogen have? Ask4Essay Why Does Nitrogen Have A Lone Pair It can donate this electron pair to form a coordinate bond. I'll give you a simple example. As with so 2, this composite model of electron distribution and negative electrostatic potential in ammonia shows that a lone pair of electrons occupies a larger region of space around the nitrogen atom than does a bonding pair of electrons that is shared. Why Does Nitrogen Have A Lone Pair.
From teachthemechanism.com
Lone Pairs and Aromaticity Teach the Mechanism Why Does Nitrogen Have A Lone Pair The lone pair electrons on the nitrogen are contained in the last sp 3 hybridized orbital. These three bonds involve three of nitrogen’s valence electrons; As with so 2, this composite model of electron distribution and negative electrostatic potential in ammonia shows that a lone pair of electrons occupies a larger region of space around the nitrogen atom than does. Why Does Nitrogen Have A Lone Pair.
From madoverchemistry.wordpress.com
62.CHEMICAL BONDING (9) Covalent Bonding(8) sp3 Hybridization Why Does Nitrogen Have A Lone Pair Have a look at the lewis structure of the. The nitrogen with a double bond forms two σ bonds with two of its sp 2 orbitals and uses the third to accommodate its lone pair. The other is involved in a pi bond, and. This coordinate bond that nitrogen forms by donating its. In ammonia the nitrogen atom is bonded. Why Does Nitrogen Have A Lone Pair.
From www.youtube.com
How to Find Lone Pairs Of Electrons of Any Atom Trick to Find Lone Why Does Nitrogen Have A Lone Pair And no, it does not break up its lone pair. Nitrogen's maximum covalency is indeed $4$. Have a look at the lewis structure of the. The remaining two valence electrons occupy a non. It can donate this electron pair to form a coordinate bond. These three bonds involve three of nitrogen’s valence electrons; This coordinate bond that nitrogen forms by. Why Does Nitrogen Have A Lone Pair.
From www.chegg.com
Solved The lone pairs on nitrogen in the following compound Why Does Nitrogen Have A Lone Pair Nitrogen's maximum covalency is indeed $4$. These three bonds involve three of nitrogen’s valence electrons; The lone pair electrons on the nitrogen are contained in the last sp 3 hybridized orbital. As with so 2, this composite model of electron distribution and negative electrostatic potential in ammonia shows that a lone pair of electrons occupies a larger region of space. Why Does Nitrogen Have A Lone Pair.
From www.numerade.com
SOLVED In what type of orbital does the lone pair on nitrogen reside Why Does Nitrogen Have A Lone Pair This coordinate bond that nitrogen forms by donating its. Nitrogen's maximum covalency is indeed $4$. The other is involved in a pi bond, and. Have a look at the lewis structure of the. I'll give you a simple example. The nitrogen with a double bond forms two σ bonds with two of its sp 2 orbitals and uses the third. Why Does Nitrogen Have A Lone Pair.
From www.youtube.com
Number of Lone Pairs and Bonding Pairs for H2O (Water) YouTube Why Does Nitrogen Have A Lone Pair Have a look at the lewis structure of the. I'll give you a simple example. The lone pair electrons on the nitrogen are contained in the last sp 3 hybridized orbital. As with so 2, this composite model of electron distribution and negative electrostatic potential in ammonia shows that a lone pair of electrons occupies a larger region of space. Why Does Nitrogen Have A Lone Pair.
From www.toppr.com
In NO3^ ion, the number of bond pair and lone pair of electrons on Why Does Nitrogen Have A Lone Pair The other is involved in a pi bond, and. These three bonds involve three of nitrogen’s valence electrons; The remaining two valence electrons occupy a non. Nitrogen has one lone pair of electrons in its 2s orbital. The lone pair electrons on the nitrogen are contained in the last sp 3 hybridized orbital. I'll give you a simple example. Have. Why Does Nitrogen Have A Lone Pair.
From www.youtube.com
delocalized vs localized lone pairs YouTube Why Does Nitrogen Have A Lone Pair These three bonds involve three of nitrogen’s valence electrons; The lone pair electrons on the nitrogen are contained in the last sp 3 hybridized orbital. And no, it does not break up its lone pair. Have a look at the lewis structure of the. In ammonia the nitrogen atom is bonded to three hydrogen atoms. The nitrogen with a double. Why Does Nitrogen Have A Lone Pair.
From www.slideserve.com
PPT Structure and Bonding PowerPoint Presentation, free download ID Why Does Nitrogen Have A Lone Pair It can donate this electron pair to form a coordinate bond. In ammonia the nitrogen atom is bonded to three hydrogen atoms. The nitrogen with a double bond forms two σ bonds with two of its sp 2 orbitals and uses the third to accommodate its lone pair. This coordinate bond that nitrogen forms by donating its. As with so. Why Does Nitrogen Have A Lone Pair.
From byjus.com
in NO3 ion number of bond pair and lone pair of electrons in nitrogen Why Does Nitrogen Have A Lone Pair And no, it does not break up its lone pair. The lone pair electrons on the nitrogen are contained in the last sp 3 hybridized orbital. This coordinate bond that nitrogen forms by donating its. Nitrogen has one lone pair of electrons in its 2s orbital. The remaining two valence electrons occupy a non. Due to the sp 3 hybridization. Why Does Nitrogen Have A Lone Pair.
From www.doubtnut.com
In NO(3)^()ion, the number of bond pairs and lone pairs of electrons Why Does Nitrogen Have A Lone Pair The nitrogen with a double bond forms two σ bonds with two of its sp 2 orbitals and uses the third to accommodate its lone pair. In ammonia the nitrogen atom is bonded to three hydrogen atoms. Nitrogen's maximum covalency is indeed $4$. I'll give you a simple example. Have a look at the lewis structure of the. These three. Why Does Nitrogen Have A Lone Pair.
From www.chegg.com
Solved Which nitrogens have “true” lone pairs?Which nitrogen Why Does Nitrogen Have A Lone Pair Due to the sp 3 hybridization the nitrogen has a. These three bonds involve three of nitrogen’s valence electrons; I'll give you a simple example. The other is involved in a pi bond, and. The nitrogen with a double bond forms two σ bonds with two of its sp 2 orbitals and uses the third to accommodate its lone pair.. Why Does Nitrogen Have A Lone Pair.
From www.masterorganicchemistry.com
Rules for Aromaticity The 4 Key Factors Master Organic Chemistry Why Does Nitrogen Have A Lone Pair In ammonia the nitrogen atom is bonded to three hydrogen atoms. This coordinate bond that nitrogen forms by donating its. I'll give you a simple example. The nitrogen with a double bond forms two σ bonds with two of its sp 2 orbitals and uses the third to accommodate its lone pair. Have a look at the lewis structure of. Why Does Nitrogen Have A Lone Pair.
From www.masterorganicchemistry.com
Evaluating Resonance Structures With Positive Charge Why Does Nitrogen Have A Lone Pair I'll give you a simple example. These three bonds involve three of nitrogen’s valence electrons; Nitrogen's maximum covalency is indeed $4$. Nitrogen has one lone pair of electrons in its 2s orbital. The nitrogen with a double bond forms two σ bonds with two of its sp 2 orbitals and uses the third to accommodate its lone pair. The lone. Why Does Nitrogen Have A Lone Pair.
From www.teachoo.com
How to find Valency? What are valence electrons? Teachoo Why Does Nitrogen Have A Lone Pair The remaining two valence electrons occupy a non. Nitrogen's maximum covalency is indeed $4$. And no, it does not break up its lone pair. The other is involved in a pi bond, and. These three bonds involve three of nitrogen’s valence electrons; Nitrogen has one lone pair of electrons in its 2s orbital. It can donate this electron pair to. Why Does Nitrogen Have A Lone Pair.
From www.youtube.com
How many bonds does Nitrogen form? YouTube Why Does Nitrogen Have A Lone Pair And no, it does not break up its lone pair. The nitrogen with a double bond forms two σ bonds with two of its sp 2 orbitals and uses the third to accommodate its lone pair. The other is involved in a pi bond, and. This coordinate bond that nitrogen forms by donating its. Nitrogen's maximum covalency is indeed $4$.. Why Does Nitrogen Have A Lone Pair.
From www.youtube.com
Number of Lone Pairs and Bonding Pairs for NO3 (Nitrate ion) YouTube Why Does Nitrogen Have A Lone Pair Nitrogen has one lone pair of electrons in its 2s orbital. This coordinate bond that nitrogen forms by donating its. As with so 2, this composite model of electron distribution and negative electrostatic potential in ammonia shows that a lone pair of electrons occupies a larger region of space around the nitrogen atom than does a bonding pair of electrons. Why Does Nitrogen Have A Lone Pair.
From www.slideserve.com
PPT The Nitrogen Cycle PowerPoint Presentation, free download ID899587 Why Does Nitrogen Have A Lone Pair It can donate this electron pair to form a coordinate bond. The remaining two valence electrons occupy a non. This coordinate bond that nitrogen forms by donating its. Nitrogen's maximum covalency is indeed $4$. As with so 2, this composite model of electron distribution and negative electrostatic potential in ammonia shows that a lone pair of electrons occupies a larger. Why Does Nitrogen Have A Lone Pair.
From school.careers360.com
How Many Lone Pairs Does Oxygen Have Why Does Nitrogen Have A Lone Pair Have a look at the lewis structure of the. And no, it does not break up its lone pair. The lone pair electrons on the nitrogen are contained in the last sp 3 hybridized orbital. It can donate this electron pair to form a coordinate bond. The remaining two valence electrons occupy a non. Due to the sp 3 hybridization. Why Does Nitrogen Have A Lone Pair.
From www.youtube.com
How many valence electrons does nitrogen have?How to find the valence Why Does Nitrogen Have A Lone Pair This coordinate bond that nitrogen forms by donating its. In ammonia the nitrogen atom is bonded to three hydrogen atoms. As with so 2, this composite model of electron distribution and negative electrostatic potential in ammonia shows that a lone pair of electrons occupies a larger region of space around the nitrogen atom than does a bonding pair of electrons. Why Does Nitrogen Have A Lone Pair.