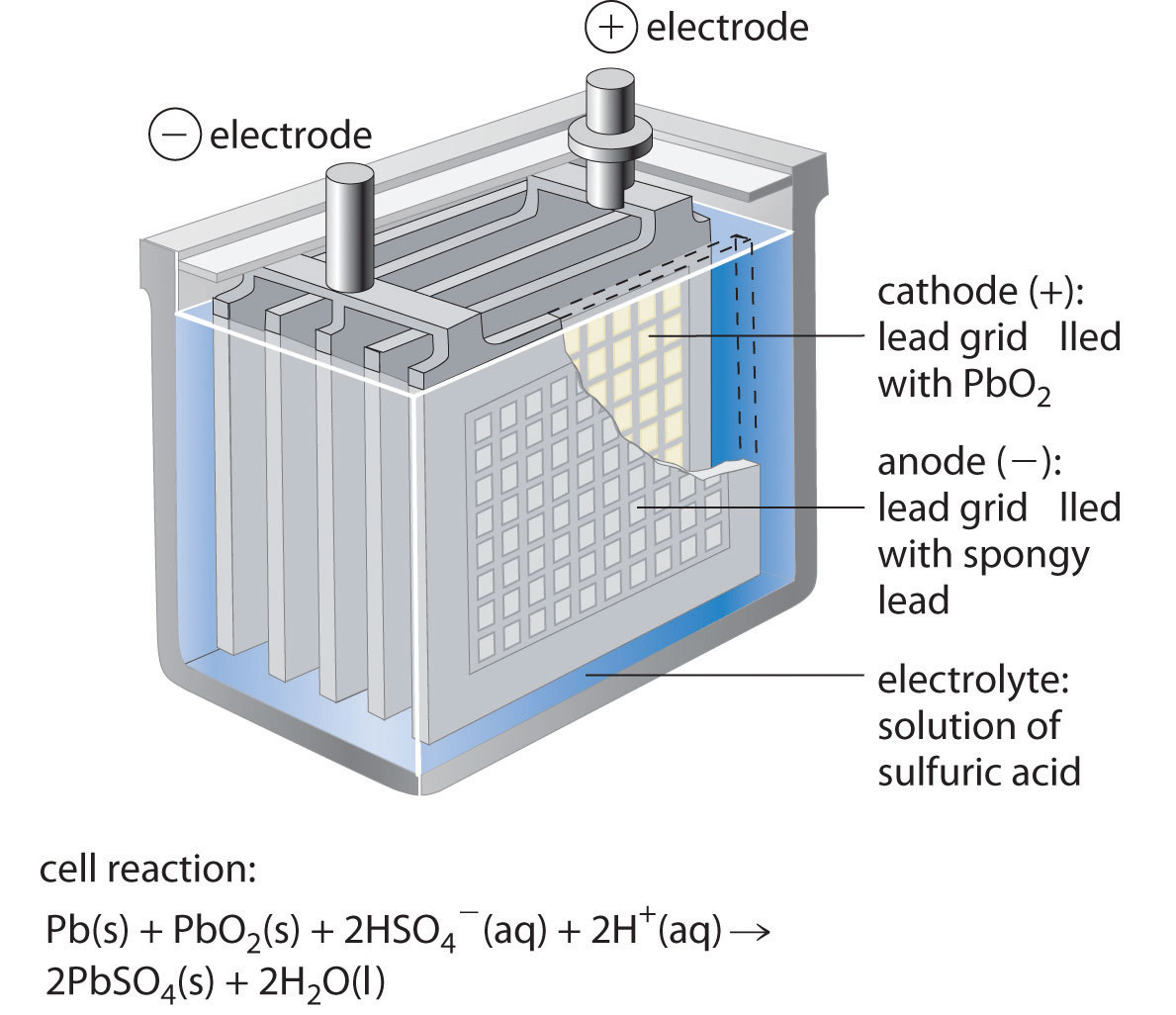
What Is A Battery In Electrochemistry . It has two terminals, an anode. electrochemical processes in batteries occur in conjunction with a spontaneous reduction in gibbs free energy resulting from differences in lattice cohesive energies and ionization free energies (in water) of reactants and products, as confirmed quantitatively for many combinations of metals. a battery is an electrochemical cell or series of cells that produces an electric current. batteries consist of two electrical terminals called the cathode and the anode, separated by a chemical material called an. A battery can be made up of one or several (like in volta's original pile) electrochemical cells. This is known as electrochemistry and the system that underpins a battery is called an electrochemical cell. Batteries power our lives by transforming energy from one type to another. what is a battery? batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical. In principle, any galvanic cell could be used. a battery is a device that stores chemical energy, and converts it to electricity. a battery is a device that stores chemical energy and converts it into electricity. a battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a constant external.
from saylordotorg.github.io
It has two terminals, an anode. batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical. batteries consist of two electrical terminals called the cathode and the anode, separated by a chemical material called an. a battery is a device that stores chemical energy, and converts it to electricity. This is known as electrochemistry and the system that underpins a battery is called an electrochemical cell. In principle, any galvanic cell could be used. a battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a constant external. electrochemical processes in batteries occur in conjunction with a spontaneous reduction in gibbs free energy resulting from differences in lattice cohesive energies and ionization free energies (in water) of reactants and products, as confirmed quantitatively for many combinations of metals. Batteries power our lives by transforming energy from one type to another. A battery can be made up of one or several (like in volta's original pile) electrochemical cells.
Electrochemistry
What Is A Battery In Electrochemistry It has two terminals, an anode. A battery can be made up of one or several (like in volta's original pile) electrochemical cells. a battery is an electrochemical cell or series of cells that produces an electric current. a battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a constant external. This is known as electrochemistry and the system that underpins a battery is called an electrochemical cell. batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical. It has two terminals, an anode. In principle, any galvanic cell could be used. what is a battery? electrochemical processes in batteries occur in conjunction with a spontaneous reduction in gibbs free energy resulting from differences in lattice cohesive energies and ionization free energies (in water) of reactants and products, as confirmed quantitatively for many combinations of metals. Batteries power our lives by transforming energy from one type to another. a battery is a device that stores chemical energy, and converts it to electricity. batteries consist of two electrical terminals called the cathode and the anode, separated by a chemical material called an. a battery is a device that stores chemical energy and converts it into electricity.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME What Is A Battery In Electrochemistry a battery is an electrochemical cell or series of cells that produces an electric current. a battery is a device that stores chemical energy and converts it into electricity. This is known as electrochemistry and the system that underpins a battery is called an electrochemical cell. a battery is a contained unit that produces electricity, whereas a. What Is A Battery In Electrochemistry.
From www.researchgate.net
Schematic representation of a Liion battery cell. Download What Is A Battery In Electrochemistry electrochemical processes in batteries occur in conjunction with a spontaneous reduction in gibbs free energy resulting from differences in lattice cohesive energies and ionization free energies (in water) of reactants and products, as confirmed quantitatively for many combinations of metals. a battery is a device that stores chemical energy, and converts it to electricity. what is a. What Is A Battery In Electrochemistry.
From www.electricity-magnetism.org
Composition of Battery Parts of Battery Electricity What Is A Battery In Electrochemistry a battery is an electrochemical cell or series of cells that produces an electric current. Batteries power our lives by transforming energy from one type to another. batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical. In principle, any galvanic cell could be used. A battery can be made up. What Is A Battery In Electrochemistry.
From www.youtube.com
Batteries Electrochemistry Chemistry Class 12 PUC YouTube What Is A Battery In Electrochemistry a battery is an electrochemical cell or series of cells that produces an electric current. It has two terminals, an anode. In principle, any galvanic cell could be used. a battery is a device that stores chemical energy, and converts it to electricity. what is a battery? batteries consist of two electrical terminals called the cathode. What Is A Battery In Electrochemistry.
From www.slideserve.com
PPT Ch. 20 Electrochemistry PowerPoint Presentation, free download What Is A Battery In Electrochemistry batteries consist of two electrical terminals called the cathode and the anode, separated by a chemical material called an. electrochemical processes in batteries occur in conjunction with a spontaneous reduction in gibbs free energy resulting from differences in lattice cohesive energies and ionization free energies (in water) of reactants and products, as confirmed quantitatively for many combinations of. What Is A Battery In Electrochemistry.
From www.slideserve.com
PPT Electrochemistry Battery cells PowerPoint Presentation, free What Is A Battery In Electrochemistry electrochemical processes in batteries occur in conjunction with a spontaneous reduction in gibbs free energy resulting from differences in lattice cohesive energies and ionization free energies (in water) of reactants and products, as confirmed quantitatively for many combinations of metals. In principle, any galvanic cell could be used. A battery can be made up of one or several (like. What Is A Battery In Electrochemistry.
From www.sliderbase.com
Electrochemistry Presentation Chemistry What Is A Battery In Electrochemistry A battery can be made up of one or several (like in volta's original pile) electrochemical cells. It has two terminals, an anode. batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical. a battery is an electrochemical cell or series of cells that produces an electric current. batteries consist. What Is A Battery In Electrochemistry.
From thechemistrynotes.com
Cell or Battery Definition,Types, and Applications What Is A Battery In Electrochemistry A battery can be made up of one or several (like in volta's original pile) electrochemical cells. a battery is an electrochemical cell or series of cells that produces an electric current. a battery is a device that stores chemical energy, and converts it to electricity. batteries consist of one or more electrochemical cells that store chemical. What Is A Battery In Electrochemistry.
From www.pinterest.com
Electrochemistry Electrochemistry, Chemistry lessons, Chemistry What Is A Battery In Electrochemistry This is known as electrochemistry and the system that underpins a battery is called an electrochemical cell. A battery can be made up of one or several (like in volta's original pile) electrochemical cells. batteries consist of two electrical terminals called the cathode and the anode, separated by a chemical material called an. batteries consist of one or. What Is A Battery In Electrochemistry.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells What Is A Battery In Electrochemistry electrochemical processes in batteries occur in conjunction with a spontaneous reduction in gibbs free energy resulting from differences in lattice cohesive energies and ionization free energies (in water) of reactants and products, as confirmed quantitatively for many combinations of metals. a battery is a device that stores chemical energy and converts it into electricity. a battery is. What Is A Battery In Electrochemistry.
From www.slideserve.com
PPT Electrochemistry Batteries PowerPoint Presentation, free download What Is A Battery In Electrochemistry electrochemical processes in batteries occur in conjunction with a spontaneous reduction in gibbs free energy resulting from differences in lattice cohesive energies and ionization free energies (in water) of reactants and products, as confirmed quantitatively for many combinations of metals. batteries consist of two electrical terminals called the cathode and the anode, separated by a chemical material called. What Is A Battery In Electrochemistry.
From ourfuture.energy
Salt Water Lamp OurFuture.Energy What Is A Battery In Electrochemistry a battery is a device that stores chemical energy, and converts it to electricity. It has two terminals, an anode. In principle, any galvanic cell could be used. a battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a constant external. a battery is an electrochemical cell or. What Is A Battery In Electrochemistry.
From www.youtube.com
Electrochemical Reaction in Lithium Ion Battery YouTube What Is A Battery In Electrochemistry In principle, any galvanic cell could be used. what is a battery? a battery is an electrochemical cell or series of cells that produces an electric current. Batteries power our lives by transforming energy from one type to another. a battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that. What Is A Battery In Electrochemistry.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME What Is A Battery In Electrochemistry what is a battery? a battery is a device that stores chemical energy and converts it into electricity. a battery is a device that stores chemical energy, and converts it to electricity. It has two terminals, an anode. A battery can be made up of one or several (like in volta's original pile) electrochemical cells. electrochemical. What Is A Battery In Electrochemistry.
From www.thoughtco.com
Electrochemical Cell Definition What Is A Battery In Electrochemistry batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical. batteries consist of two electrical terminals called the cathode and the anode, separated by a chemical material called an. a battery is an electrochemical cell or series of cells that produces an electric current. a battery is a contained. What Is A Battery In Electrochemistry.
From www.researchgate.net
Schematic of lithium ion battery electrochemical model.... Download What Is A Battery In Electrochemistry a battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a constant external. It has two terminals, an anode. a battery is a device that stores chemical energy, and converts it to electricity. batteries consist of one or more electrochemical cells that store chemical energy for later conversion. What Is A Battery In Electrochemistry.
From www.slideserve.com
PPT Electrochemistry Batteries PowerPoint Presentation, free download What Is A Battery In Electrochemistry batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical. a battery is a device that stores chemical energy, and converts it to electricity. a battery is a device that stores chemical energy and converts it into electricity. This is known as electrochemistry and the system that underpins a battery. What Is A Battery In Electrochemistry.
From saylordotorg.github.io
Electrochemistry What Is A Battery In Electrochemistry electrochemical processes in batteries occur in conjunction with a spontaneous reduction in gibbs free energy resulting from differences in lattice cohesive energies and ionization free energies (in water) of reactants and products, as confirmed quantitatively for many combinations of metals. a battery is a device that stores chemical energy and converts it into electricity. a battery is. What Is A Battery In Electrochemistry.
From generic.wordpress.soton.ac.uk
Electrochemistry explanations, videos and everyday life examples What Is A Battery In Electrochemistry a battery is a device that stores chemical energy and converts it into electricity. batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical. A battery can be made up of one or several (like in volta's original pile) electrochemical cells. It has two terminals, an anode. a battery is. What Is A Battery In Electrochemistry.
From pubs.acs.org
How Batteries Store and Release Energy Explaining Basic What Is A Battery In Electrochemistry batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical. a battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a constant external. It has two terminals, an anode. a battery is a device that stores chemical energy, and converts it. What Is A Battery In Electrochemistry.
From pubs.acs.org
How Batteries Store and Release Energy Explaining Basic What Is A Battery In Electrochemistry batteries consist of two electrical terminals called the cathode and the anode, separated by a chemical material called an. a battery is a device that stores chemical energy, and converts it to electricity. what is a battery? This is known as electrochemistry and the system that underpins a battery is called an electrochemical cell. It has two. What Is A Battery In Electrochemistry.
From usermanualtractors.z1.web.core.windows.net
What Happens At The Cathode In Electrolysis What Is A Battery In Electrochemistry a battery is an electrochemical cell or series of cells that produces an electric current. Batteries power our lives by transforming energy from one type to another. a battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a constant external. In principle, any galvanic cell could be used. . What Is A Battery In Electrochemistry.
From www.sigmaaldrich.com
Electrochemistry on the Bench and in the Field What Is A Battery In Electrochemistry a battery is a device that stores chemical energy and converts it into electricity. a battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a constant external. This is known as electrochemistry and the system that underpins a battery is called an electrochemical cell. It has two terminals, an. What Is A Battery In Electrochemistry.
From cpb.iphy.ac.cn
Brief overview of electrochemical potential in lithium ion batteries What Is A Battery In Electrochemistry Batteries power our lives by transforming energy from one type to another. batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical. electrochemical processes in batteries occur in conjunction with a spontaneous reduction in gibbs free energy resulting from differences in lattice cohesive energies and ionization free energies (in water) of. What Is A Battery In Electrochemistry.
From 2012books.lardbucket.org
Electrochemistry What Is A Battery In Electrochemistry electrochemical processes in batteries occur in conjunction with a spontaneous reduction in gibbs free energy resulting from differences in lattice cohesive energies and ionization free energies (in water) of reactants and products, as confirmed quantitatively for many combinations of metals. batteries consist of two electrical terminals called the cathode and the anode, separated by a chemical material called. What Is A Battery In Electrochemistry.
From saylordotorg.github.io
Electrochemistry What Is A Battery In Electrochemistry A battery can be made up of one or several (like in volta's original pile) electrochemical cells. a battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a constant external. batteries consist of two electrical terminals called the cathode and the anode, separated by a chemical material called an.. What Is A Battery In Electrochemistry.
From school.careers360.com
Electrochemical Cell Overview, Structure, Properties & Uses What Is A Battery In Electrochemistry In principle, any galvanic cell could be used. A battery can be made up of one or several (like in volta's original pile) electrochemical cells. batteries consist of two electrical terminals called the cathode and the anode, separated by a chemical material called an. what is a battery? This is known as electrochemistry and the system that underpins. What Is A Battery In Electrochemistry.
From www.researchgate.net
4 Schematic of the electrochemical discharging process in the What Is A Battery In Electrochemistry a battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a constant external. Batteries power our lives by transforming energy from one type to another. electrochemical processes in batteries occur in conjunction with a spontaneous reduction in gibbs free energy resulting from differences in lattice cohesive energies and ionization. What Is A Battery In Electrochemistry.
From www.britannica.com
Battery Composition, Types, & Uses Britannica What Is A Battery In Electrochemistry a battery is an electrochemical cell or series of cells that produces an electric current. Batteries power our lives by transforming energy from one type to another. a battery is a device that stores chemical energy, and converts it to electricity. batteries consist of one or more electrochemical cells that store chemical energy for later conversion to. What Is A Battery In Electrochemistry.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types What Is A Battery In Electrochemistry a battery is a device that stores chemical energy and converts it into electricity. batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical. a battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a constant external. a battery is. What Is A Battery In Electrochemistry.
From www.livescience.com
How Do Batteries Work? Live Science What Is A Battery In Electrochemistry batteries consist of two electrical terminals called the cathode and the anode, separated by a chemical material called an. A battery can be made up of one or several (like in volta's original pile) electrochemical cells. In principle, any galvanic cell could be used. This is known as electrochemistry and the system that underpins a battery is called an. What Is A Battery In Electrochemistry.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells What Is A Battery In Electrochemistry a battery is an electrochemical cell or series of cells that produces an electric current. a battery is a device that stores chemical energy, and converts it to electricity. batteries consist of two electrical terminals called the cathode and the anode, separated by a chemical material called an. This is known as electrochemistry and the system that. What Is A Battery In Electrochemistry.
From bmspowersafe.com
Electrochemistry in a battery a strategic choice What Is A Battery In Electrochemistry In principle, any galvanic cell could be used. It has two terminals, an anode. A battery can be made up of one or several (like in volta's original pile) electrochemical cells. what is a battery? a battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a constant external. . What Is A Battery In Electrochemistry.
From classnotes.org.in
Batteries Chemistry, Class 12, Electro Chemistry What Is A Battery In Electrochemistry batteries consist of two electrical terminals called the cathode and the anode, separated by a chemical material called an. a battery is an electrochemical cell or series of cells that produces an electric current. This is known as electrochemistry and the system that underpins a battery is called an electrochemical cell. A battery can be made up of. What Is A Battery In Electrochemistry.
From www.youtube.com
How to Make a Battery From Voltaic Pile 🔋 Electrochemistry 🚥 YouTube What Is A Battery In Electrochemistry In principle, any galvanic cell could be used. A battery can be made up of one or several (like in volta's original pile) electrochemical cells. batteries consist of two electrical terminals called the cathode and the anode, separated by a chemical material called an. a battery is a device that stores chemical energy and converts it into electricity.. What Is A Battery In Electrochemistry.